Sulfuryl Fluoride
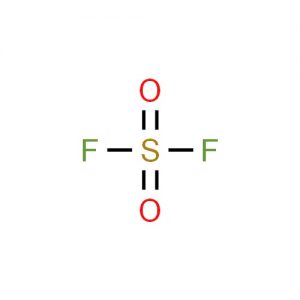
Sulfuryl fluoride represented by the chemical formula SO2F2 or F2O2S that bears the IUPAC name sulfuryl difluoride is a colorless and odorless compressed or liquefied gas. It is a sulfuryl halide that is stable in light, non-corrosive to metals and thermally stable up to 400oC when it is dry [1, 3].
Sulfuryl Fluoride Identification |
|
| CAS Number | 2699-79-8 [1] |
| PubChem CID | 17607 [1] |
| ChemSpider ID | 16647 [4] |
| EC Number | 220-281-5 [1] |
How to Make It
Sulfuryl fluoride can be prepared from an anhydrous gaseous mixture of chlorine, sulfur dioxide and hydrofluoric acid heated to 35oC in the presence of charcoal catalyst [6].
SO2 + Cl2 + 2HF = SO2F2 + 2HCl
Properties and Characteristics of Sulfuryl Fluoride
General Properties |
|
| Molar mass/molecular weight | 102.055 g/mol [1] |
Physical Properties |
|
| Color/appearance | Colorless [1] |
| Melting point/freezing point | -135.8°C, -212.44°F [2] |
| Boiling point | -55.3°C, -67.54°F [2] |
| Density | 3.72 g l-1 [2] |
| Vapor pressure | 1.7X103 kPa at 21.1oC [2] |
| State of matter at room temperature (normal phase) | Gas [1] |
Chemical Properties |
|
| Solubility in water | 4-5 ml/100 ml [2] |
| pH | 7 [3] |
Sulfuryl Fluoride (SO2F2) Uses
- As a fumigant insecticide for wooden structures to prevent the spread of termites, bed bugs and wood-infesting beetles [1].
- In organic synthesis [1].
- In making pesticides for plants [1].
Is It Dangerous
If inhaled, the gas is severely irritating to the respiratory tract and may cause lung edema. It may have a harmful effect (depression) on the central nervous system resulting in respiratory failure and convulsions. The rapid evaporation of its liquid form results in frostbite. It causes redness of eyes. Exposure of the poisonous gas could be a possible reason for even death [2]. As a safety measure, avoid inhalation and skin contact and do not wear rubber boots or gloves. Wear goggles and protective clothing [5].
- References
- Sulfuryl fluoride – Pubchem.ncbi.nlm.nih.gov
- Sulfuryl fluoride – Inchem.org
- Sulfuryl fluoride (Vikane) – Cdpr.ca.gov
- Sulfuryl fluoride – Chemspider.com
- Sulfuryl fluoride – Toxnet.nlm.nih.gov
- Sulfuryl fluoride (Vikane): A Review of its Use as a Fumigant – Repository.si.edu