Fehling’s Solution
Definition: What is Fehling’s Solution?
Fehling’s solution, or Fehling’s reagent, is a chemical reagent that is used to distinguish between an aldehyde and a ketone other than α-hydroxy ketone. Practically, it is used for the determination of reducing and non-reducing sugars that are present in carbohydrates. The test employed for this purpose is known as Fehling’s test. Fehling’s solution cannot be used for aromatic aldehyde [1-5].
The history of the test goes back to 1849 when German chemist Hermann von Fehling developed the reaction.
Preparation of Fehling’s Solution
Fehling’s solution is prepared by combining two separate solutions: Fehling A and Fehling B. Fehling A is a blue-colored aqueous solution of copper (II) sulfate (CuSO4). Fehling B is a colorless aqueous solution of potassium sodium tartrate (KNaC4H4O6·4H2O, also known as Rochelle salt) in an alkaline base like sodium hydroxide (NaOH). The two solutions are individually prepared and later mixed to give Fehling’s solution, which is blue. In this final mixture, aqueous tartrate ions from the dissolved Rochelle salt bond to Cu2+ (aq) ions from the dissolved copper sulfate crystals as bidentate ligands, giving a bistartratocuprate (II) complex [1-5].
Principle of Fehling’s Test
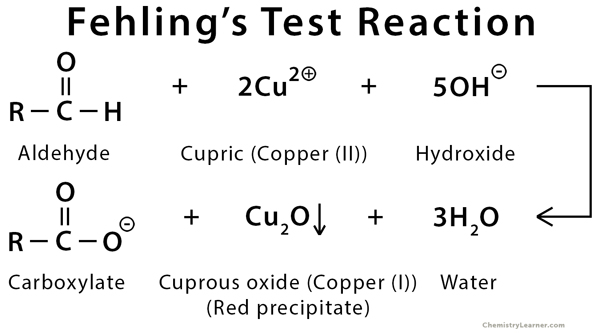
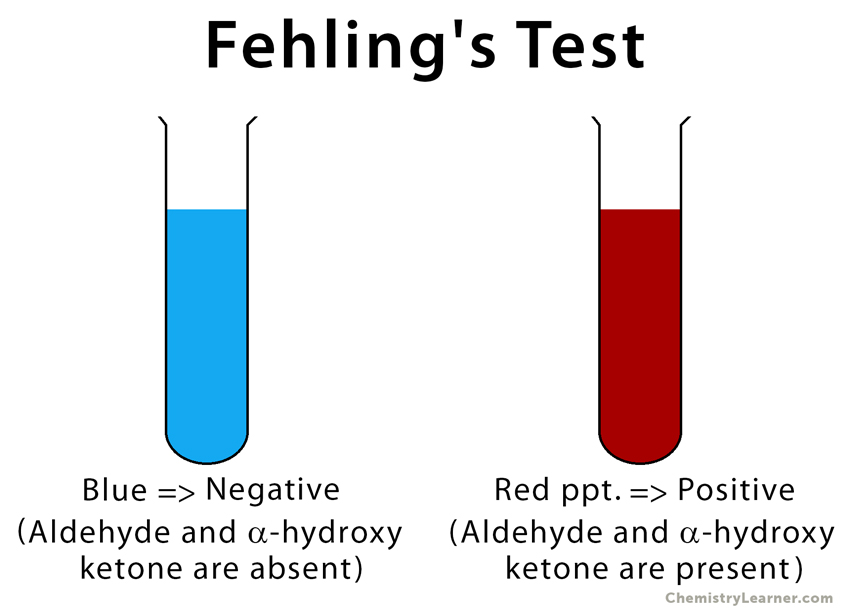
The principle of Fehling’s test is similar to that of Benedict’s test. When aldehydes are added to Fehling’s solution, they are easily oxidized by the bistartratocuprate (II) complex. During this process, copper (II) ions are reduced to copper (I) ions, leaving a red precipitate of copper (I) oxide (Cu2O). The presence of red precipitate indicates a positive result [6,7].
Example of Fehling’s Test
Result of Fehling’s Test
Mechanism of Fehling’s Test [8]
Uses and Applications of Fehling’s Solution
Fehling’s solution is used to test for monosaccharides. The most important application is to detect reducing sugars like glucose. Excess glucose in blood and urine can lead to diabetes. Fehling’s reagent is also used to break down starch into glucose syrup and maltodextrins, a polysaccharide used as a food additive [1].
FAQs
Ans. Benzaldehyde does not give Fehling’s test because it does not contain an alpha hydrogen atom.
Ans. Yes. Fehling’s test can be used for formaldehyde.
Ans. Fehling’s solutions A and B are kept separate because if they are combined, the bistartratocuprate (II) complex that is formed will quickly degrade.
Ans. Sucrose does not reduce Fehling’s solution because it lacks a free aldehyde or ketone group.
Ans. Aldehyde is more reactive towards nucleophilic addition reaction than ketone because of its stereochemistry and electronic properties. Unlike ketone, aldehyde has single hydrogen on one side of the carbonyl functional group, which makes it easier for a nucleophile to attack.
References
- Definition and preparation – Byjus.com
- Definition and preparation – Byjus.com
- Definition and preparation – Chemdemos.uoregon.edu
- Definition – Chemguide.co.uk
- Definition – Chem.ucla.edu
- Principle – Masterorganicchemistry.com
- Principle – Toppr.com
- Mechanism – Masterorganicchemistry.com


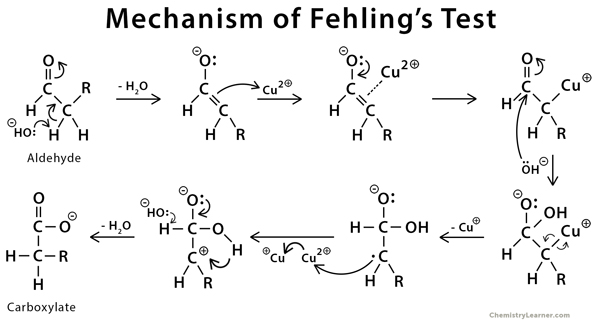





At the end carbon #2 contain an additional H ..from where it is come..????
It comes from the -OH group. We have updated the image. Thank you for bringing it to our attention.
Sir there is ambiguity in second last step of mechanism or it is not properly written
We will look into it. Thank you.
Answer of question 1 is wrong.pls make it correct.
Thank you. We have edited the response.