Metallic Bond
What is Metallic Bonding?
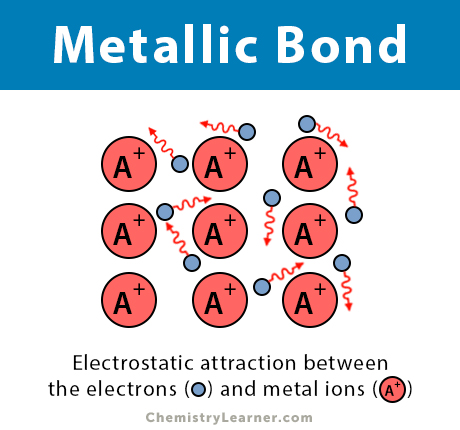
A metallic bond is a type of chemical bond in which a ‘cloud’ of free moving valence electrons is bonded to the positively charged ions in a metal. It can be described as the sharing of free electrons among a lattice of positively charged metal ions. The structure of metallic bonds is entirely different from that of ionic and covalent bonds. Metal is the only substance that contains a metallic bond [1-5].
German physicist Paul Drude first introduced the idea of metallic bonding in 1900.
How are Metallic Bonds Formed
The electrons are detached from the atoms and delocalized throughout the metal, i.e., they move freely. However, the interactions between the ions and electrons are still prevalent. These interactions give rise to a binding force that holds the metallic crystal together. This force is the basis of a metallic bond [1-5].
Properties and Characteristics of Metallic Bond
The metallic bond is responsible for many of the properties of metals [1,2].
Electrical and thermal conductivity: The mobile electrons are charge carriers in the conduction of electricity and energy carriers in heat conduction. Therefore, metals can conduct electricity and heat.
Malleability and ductility: A metal can be hammered into sheets and drawn into wires. These shapes are possible because the atoms share electrons and slide past each other.
High melting and boiling points: The metallic bond is formed due to the strong electrostatic forces between the sea of electrons and cations. As a result, metals have high melting and boiling points.
Luster and high reflectivity: The delocalized electrons willingly absorb and re-emit visible light. This property gives metals their characteristic luster.
Examples of Metallic Bond
The metallic bond is commonly observed in metals. Here are some examples [2-4]:
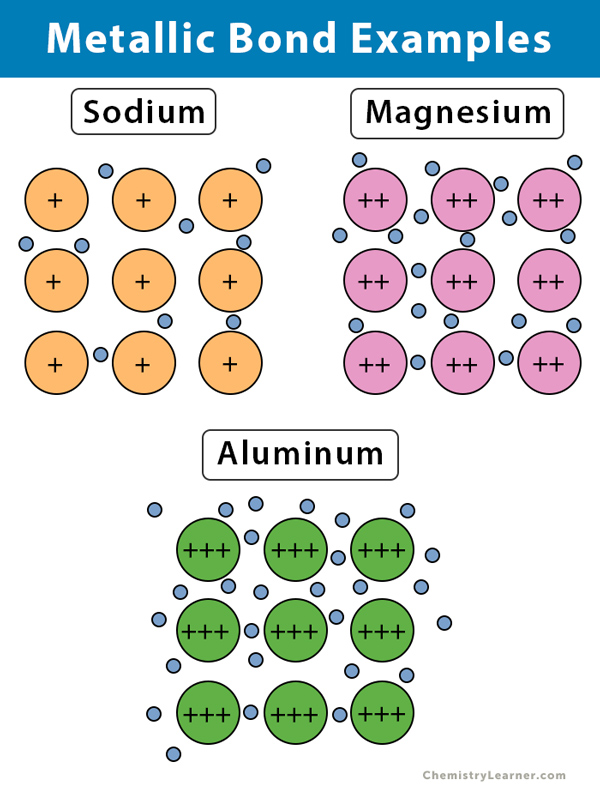
1. Sodium (Na)
Sodium has a lone electron in its outermost orbital, i.e., the 3s orbital. When sodium atoms arrange together, the outermost electron of one atom shares space with the corresponding electron on a neighboring atom. As a result, a 3s molecular orbital is formed. Each sodium atom has eight other atoms in its neighbor. The sharing takes place between a central sodium atom and the 3s orbital of its neighbors.
All the 3s orbitals overlap to give many molecular orbitals that extend over the entire sodium metal. The outermost electrons are said to be delocalized over the whole metal structure. These electrons are no longer attached to any particular atom but move freely around the entire metal.
2. Magnesium (Mg)
Magnesium has two electrons in its outermost shell, the 3s shell. Both these electrons are delocalized. The metallic bond formation in magnesium is the same as sodium, except it has more electron density than sodium. Besides, each of magnesium nucleus has a twice the charge as in sodium. Therefore, the attraction between the nuclei and the delocalized electrons will be stronger than sodium. The strength of the bond is generally higher in magnesium.
3. Aluminum (Al)
Aluminum has three valence electrons in the 3s orbital. When the atoms lose all three electrons, aluminum ions end up having a positive charge +3. These positively charged ions repel each other but are held together in the block by the negative electrons. As a result, by sharing the electrons, the cations arrange themselves in a steady pattern. This regular pattern of atoms gives rise to the crystalline structure of metals. In a crystal lattice, atoms are tightly packed close to one another to maximize the bond strength.
FAQs
Ans. Some metals are soluble in water, whereas others are not. Alkali metals like sodium and potassium react vigorously react with water releasing hydrogen gas.
Ans. Yes. A covalent bond is more robust than a metallic because of the overlap of electron orbital.