Chain Reaction
What is a Chain Reaction
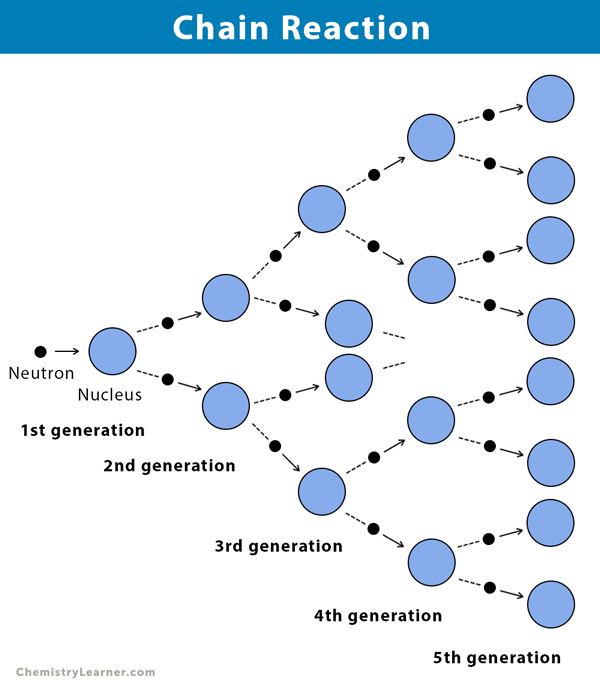
A chain reaction is a type of chemical reaction that repeats over time. The products generated in a single reaction cycle become the reactants to initiate the next reaction.
A chain reaction can be self-sustaining or controlled. The reaction will continue until the system reaches a stable state. Usually, a stable state is achieved when the fuel that initiates the reaction runs out. For example, a candle will keep burning until it runs out of wax, which is the fuel responsible for burning.
The history of the chain reaction goes back to 1930 when British physical chemist Cyril Norman Hinshelwood and Soviet physicist and chemist Nikolay Semenov developed it independently.
Steps of Chain Reaction
A chain reaction proceeds by a sequence of three steps.
- Initiation: Formation of active particles or chain carriers by light, heat, or catalyst.
- Propagation: Active particles react with the original reactants, thereby creating more active particles, which initiate the next reaction. The number of active particles created is equal to the number consumed.
- Termination: Loss of activity of the active particle due to the consumption of all the reactants, resulting in termination
Sometimes chain branching and chain inhibition also take place. In the former, the number of active particles created is more than the number consumed. In the latter, a substance is added to inhibit the reaction and not form products.
Examples of Chain Reaction
Here are some examples of the chain reaction.
1. Polymerase Chain Reaction
The real-time polymerase chain reaction (PCR) is a technique that makes a million copies of the DNA, allowing scientists to use it for genetic research, forensic analysis, and medical diagnosis.
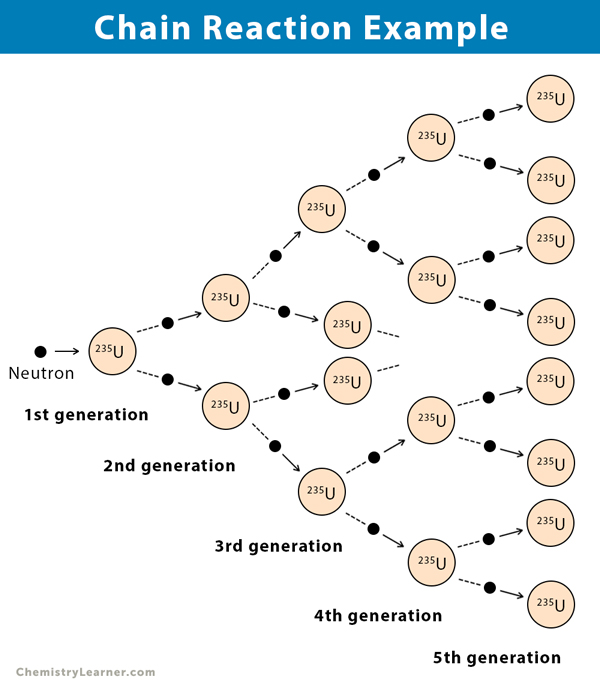
2. Nuclear Chain Reaction
The nuclear chain reaction is a series of chemical reactions where a nucleus, typically 235U, is bombarded with neutrons, thereby creating an explosion and releasing a large amount of energy. This process is also known as nuclear fission and is the principle behind the first atom bomb developed in the 1940s.
What Happens in a Chain Reaction
In a chain reaction, sometimes free radicals form as active particles. They react with alkane and alkene to form new products. The mechanism of the free radical chain reaction of polymerization of ethylene is discussed below.
Polymerization of Ethylene
During the polymerization of ethylene, many ethylene monomers combine to form a long chain of polyethylene in the presence of oxygen.
nCH2=CH2 → [-CH2-CH2-]n
Here are the different steps of the reaction.
Step 1: Initiation
The oxygen in the reaction reacts with ethene to give a peroxide, which is very unstable. The peroxide decomposes into free radicals upon reacting with light or heat. These free radicals are the chain carriers.
R-O-O-R → 2 R-O·
Step 2: Propagation
The free radical from the first steps adds to the ethene, breaks the pi-bond, and bonds firmly with one of the carbons. The remaining electron from the pi-bond is used to form a new free radical. The new product adds an ethene molecule in a chain reaction.
R-O· + CH2=CH2 → R-O-CH2-CH2·
R-O-CH2-CH2· + CH2=CH2 → R-O-CH2-CH2-CH2-CH2·
Step: Termination
The chain is terminated when two free radicals combine, and there is none left to initiate another reaction.
R-O(-CH2-CH2-)n· + ·(-CH2-CH2-)mO-R → R-O(-CH2-CH2-)n+m-O-R
Application of Chain Reaction
The chain reaction has quite a few functions and applications that are put to practical use.
- Nuclear chain reaction is used to power nuclear plants to supply electricity
- Polymerase chain reaction is used to create genetic fingerprints that are very useful in forensic analysis and crime investigation
- Sometimes a chain reaction generates radical scavengers that are used to prevent spoilage of food due to oxidation.
FAQs
Ans. The main difference between controlled and uncontrolled chain reactions is that controlled chain reactions keep the reaction under control and do not lead to any explosive effects. On the other hand, uncontrolled chain reactions lead to fire and explosion along with energy.
Ans. Chain reactions can be kept under control by introducing a boron control rod in a nuclear reactor.