Gas Chromatography
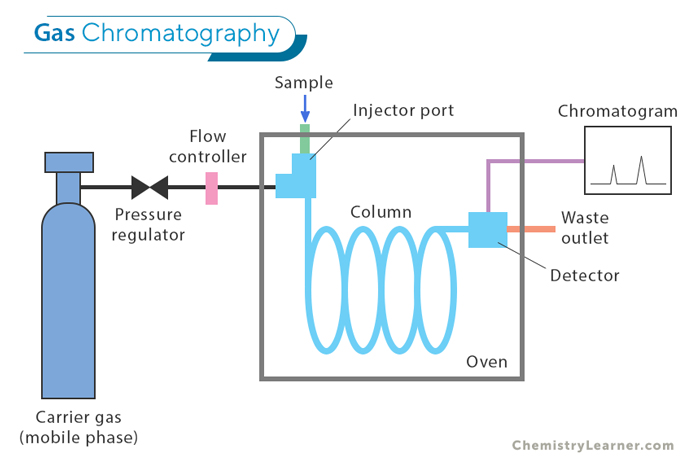
Gas chromatography (GC) is a powerful analytical technique that separates and analyzes compounds in a gas phase. The instrument used for this process consists of a sample injector, a column where separation occurs, a detector to measure compound concentrations, and a data recording system. [1-4]
The sample is vaporized and injected into the column, interacting with the stationary phase to separate its components based on their chemical properties. The separated compounds are then detected as they exit the column.
How Does Gas Chromatography Work
The separation mechanism in gas chromatography relies on the differential interaction of compounds with the stationary phase inside a column. The process involves several steps. [1-4]
First, the sample is injected into the system, vaporized, and entered into the column. As the carrier gas (usually helium or nitrogen) flows through the column, compounds interact with the stationary phase, leading to differential retention times.
Next, as compounds exit the column, they are detected by a detector, which produces signals recorded by a computer. The resulting chromatogram provides information on the sample’s retention times and concentrations.
What is Retention Time in Gas Chromatography
Retention time in gas chromatography is when a specific compound travels through a chromatographic column and reaches the detector. It is a crucial parameter to identify and quantify compounds in a sample. [1-4]
The retention time is calculated by measuring the time from injection of the sample until the compound elutes from the column and is detected. It is influenced by various factors such as column temperature, stationary phase, carrier gas flow rate, and molecular interactions between the compound and stationary phase.
Which Factors Influence the Separation of the Components
The separation of components can be influenced by various factors, such as [1-4]
1. Vapor Pressure
The relationship between a compound’s boiling point and its polarity determines its vapor pressure. Compounds with lower boiling points exhibit higher vapor pressures, resulting in shorter retention times. This is why solvents with low boiling points, such as diethyl ether and dichloromethane, are often used to dissolve samples. The column temperature does not necessarily need to exceed the boiling point, as all compounds, even solids, have a non-zero vapor pressure at any temperature.
2. Polarity of Components and Stationary Phase
The polarity of both the compound and the stationary phase plays a significant role in retention time. Similar polarities between the compound and the stationary phase lead to increased retention times due to stronger interactions. Consequently, polar compounds exhibit longer retention times on polar stationary phases and shorter times on non-polar columns.
3. Column Temperature
Excessively high temperatures result in short retention times but poor separation, as most components remain in the gas phase. Optimal separation occurs with temperature gradients, utilizing differences in polarity and boiling points. If the compound does not interact with the stationary phase, retention time decreases, and the separation quality diminishes.
4. Carrier Gas Flow Rate
The flow rate of the carrier gas impacts retention times and separation quality. A high flow rate reduces retention times but compromises separation as components have limited interaction with the stationary phase. Insufficient interaction results in poor separation as compounds rapidly push through the column.
5. Column Length
A longer column generally improves separation, but it comes with trade-offs. Longer columns proportionally increase retention time and introduce significant peak broadening due to increased longitudinal diffusion. Gas molecules move not only in one direction but also sideways and backward. Broadening is inversely proportional to the flow rate. It occurs due to finite mass transfer rates and varied paths through the column.
6. Amount of Material Injected
The injection of an appropriate amount of material is crucial for achieving symmetric peaks in the chromatogram. Excessive sample injection causes significant tailing in peaks, leading to poorer separation. Most detectors are sensitive enough to detect signals with small sample amounts. Typically, only a small percentage (1-2%) of the injected compound passes through the column under standard conditions. This is because gas chromatography instruments often operate in split mode to prevent column and detector overload.
Detectors Used in Gas Chromatography
Several types of detectors are used in gas chromatography, each with its unique function and advantages. [1-4]
The Mass Spectrometry Detector or Mass Spectrometer can provide detailed information about the molecular structure of compounds. Mass spectrometers ionize molecules and separate them based on their mass-to-charge ratio, allowing for precise identification and quantification of analytes.
The Flame Ionization Detector (FID) is sensitive to hydrocarbons and provides excellent sensitivity for organic compounds. It works by ionizing organic molecules in a hydrogen flame, producing electric currents proportional to the concentration of the analyte.
The Thermal Conductivity Detector (TCD) measures the heat changes in a carrier gas caused by the presence of analytes in the sample. This sensitive detector can detect and quantify various compounds based on their thermal conductivity properties.
The Electron Capture Detector (ECD) works by detecting electronegative compounds, such as halogenated organic compounds and pesticides, which can capture electrons released from a radioactive source within the detector. This detection method allows for identifying and quantifying trace levels of these compounds in a sample.
Applications of Gas Chromatography
Gas chromatography finds extensive applications across diverse industries and research fields. It is widely used in various industries such as pharmaceuticals, environmental analysis, food and beverage testing, etc. Its applications include: [1-4]
- Detection of pollutants in air, water, and soil
- Quality control and flavor profiling in food products
- Identification and quantification of active pharmaceutical ingredients (APIs)
- Identification of accelerants in arson investigations
- Determination of the composition of refined products like gasoline.
- Detection of metabolites and biomarkers in biological samples
- Identification and quantification of volatile compounds in perfumes and flavors
- Detection of potential allergens and contaminants in cosmetics