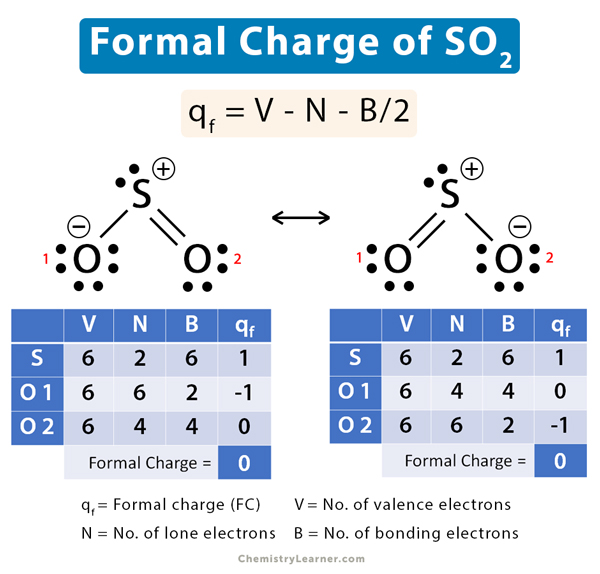
Sulfur Dioxide (SO2) Formal Charge
In sulfur dioxide (SO2), sulfur (S) has a double covalent bond with one oxygen (O) atom and a single covalent bond with another. SO2 undergoes the following resonance.
O––S+=O ↔ O=S+–O–
Let us calculate the formal charge of both these structures.
i. O––S+=O
V = 6, N = 2, B = 6
Therefore,
qf = 6 – 2 – 6/2 = 1
Oxygen 1
V = 6, N = 6, B = 2
Therefore,
qf = 6 – 6 – 2/2 = -1
Oxygen 2
V = 6, N = 4, B = 4
Therefore,
qf = 6 – 4 – 4/2 = 0
The net formal charge is: 1 – 1 + 0 = 0
ii. O=S+–O–
Sulfur
V = 6, N = 2, B = 6
Therefore,
qf = 6 – 2 – 6/2 = 1
Oxygen 1
V = 6, N = 4, B = 4
Therefore,
qf = 6 – 4 – 4/2 = 0
Oxygen 2
V = 6, N = 6, B = 2
Therefore,
qf = 6 – 6 – 2/2 = -1
The net formal charge is: 1 + 0 – 1 = 0
The formal charge of SO2 is zero. It spends half its time in each of its two resonance structures. It flips back and forth swiftly between the two forms.