Ionic Radius
What is Ionic Radius
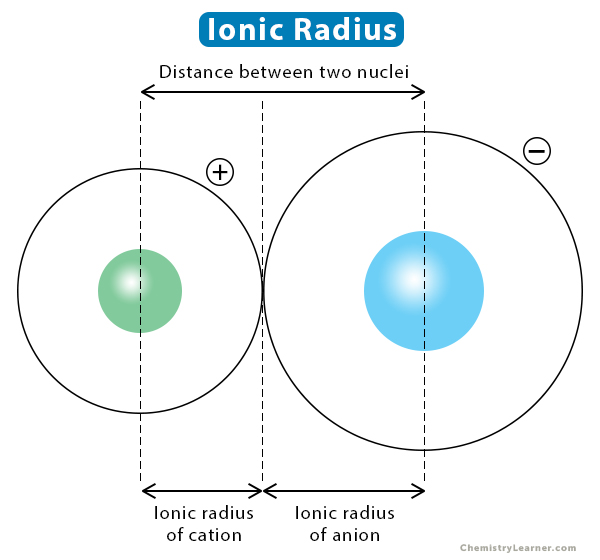
The ionic radius is the distance of the outermost shell of electrons from the nucleus of an ion. It indicates the size of an ion in a crystal lattice where two atoms are bonded by an ionic bond. The ionic radius is analogous to the atomic radius of an atom [1-4].
How to Find Ionic Radius
The ionic radius can be determined from the ionic bond between two atoms. However, the ionic radius is problematic to measure since it depends upon the environment in which the ion is located. It depends on the coordination number and spin state of the ion. The ionic radius is generally calculated by estimating the distance between the two nuclei and dividing it according to the atomic sizes. It is measured in picometer (pm) or nanometer (nm) unit [1,2].
Ionic Radius vs. Atomic Radius
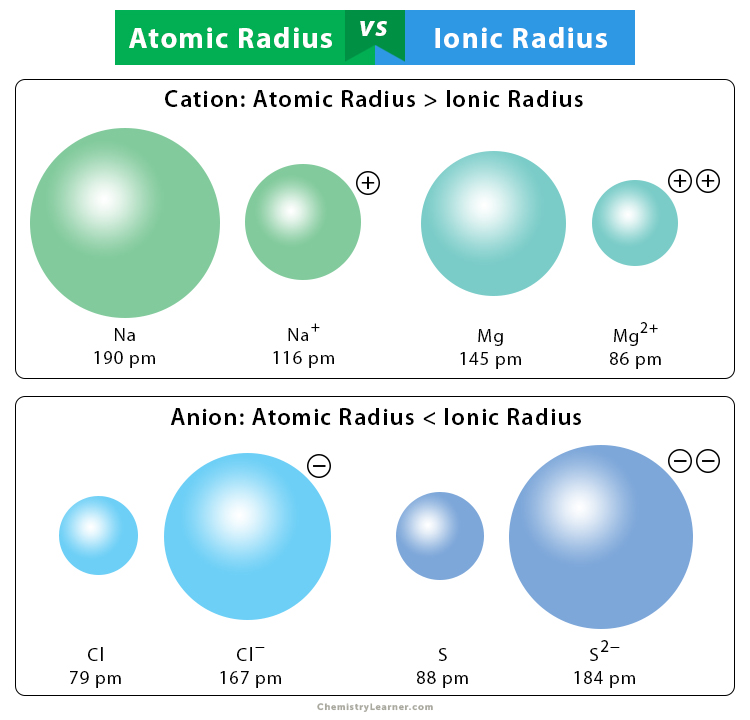
An ionic bond is formed when one atom donates electrons to the other, resulting in ions. The atom that loses electrons is known as a cation. The atom that gains electrons is known as an anion. The ionic radius of cations is generally smaller than their corresponding atomic radius. The reason is that when atoms lose valence electrons, they lose an entire electron shell that shrinks their radius. On the other hand, the ionic radius of cations is greater than their corresponding atomic radius. When electrons are added to the valence shell, they repel each other and increase the radius [1,2,7].
Examples
- The atomic radius of the sodium atom (Na) is 190 pm, and the ionic radius of sodium ion (Na+) is 116 pm.
- The atomic radius of the chlorine atom (Cl) is 79 pm, and the ionic radius of chloride ion (Cl–) is 167 pm.
Most periodic table elements, except noble gases, lose or gain electrons to form ions. Their ionic radius trend is discussed below.
Ionic Radius Trend in Periodic Table
The elements display a specific pattern in their ionic radius across a period and down a group in the periodic table. This pattern is known as the ionic radius trend [1-3,5].
Vertical: Ionic Radius Trend Down a Group
Down a group, the atomic size and the ionic radius increases for ions with the same charge, i.e., same oxidation state. The reason is that when an extra shell of electrons is added to the atom, the valence electrons get further away from the nucleus. The inner electrons shield the outer electrons, and the shielding increases down the group. An effect of shielding is that the valence electrons now see a nucleus that is reduced in charge, known as the effective nuclear charge (Zeff). As a result, the attractive electrostatic force between the nucleus and the valence electrons also decreases, and the electrons remain further away. Thus, the ionic radius has the same vertical trend as the atomic radius.
The following table gives the ionic radius values of group 1 elements for 6-coordinate, octahedral geometry [7].
| Ion | Electron configuration | Ionic radius (pm) (6-coordinate, octahedral) |
| Li+ | 2 | 90 |
| Na+ | 2, 8 | 116 |
| K+ | 2, 8, 8 | 152 |
| Rb+ | 2, 8, 18, 8 | 166 |
| Cs+ | 2, 8, 18, 18, 8 | 181 |
The following table gives the ionic radius values of group 17 elements for 6-coordinate, octahedral geometry [7].
| Ion | Electron configuration | Ionic radius (pm) (6-coordinate, octahedral) |
| Fl– | 2, 8 | 119 |
| Cl– | 2, 8, 8 | 167 |
| Br– | 2, 8, 18, 8 | 182 |
| I– | 2, 8, 18, 18, 8 | 206 |
Horizontal: Ionic Radius Trend Across a Period
Due to an atom’s unique ability to gain or lose electrons, the ionic radius trend across a period is not precisely similar to the atomic radius trend. Hence, this trend must be studied separately for cations and anions.
Consider the period 3 elements which are shown in the table below. Three cations and three anions are listed together with their number of protons, electron configuration, and ionic radius for 6-coordinate, octahedral geometry [7].
| Na+ | Mg2+ | Al3+ | P3- | S2- | Cl– | |
| Number of protons | 11 | 12 | 13 | 15 | 16 | 17 |
| Electronic configuration | 2,8 | 2,8 | 2,8 | 2,8,8 | 2,8,8 | 2,8,8 |
| Ionic radius (pm) | 116 | 86 | 67.5 | 212 | 184 | 167 |
Cations
The cations Na+, Mg2+, and Al3+ have the same electron configuration. Ions having the same electron configuration are isoelectronic. Their ionic radii decrease from left to right. The reason is that an increase in the number of protons leads to an increased attractive electrostatic force between the nucleus and the valence electrons. The electrons are held closer to the nucleus, and the radius shrinks.
Anions
The cations P3-, S2-, and Cl– are isoelectronic, and their ionic radius decreases from left to right. The reason is precisely the same. An increase in protons increases the electrostatic force and brings the electrons closer to the nucleus.
Also, from Al3+ to P3-, there is a sudden jump in the ionic radius due to an extra shell of electrons.
Trend Among Isoelectronic Ions
The ionic radius values of isoelectronic ions N3-, O2-, F–, Na+, Mg2+, and Al3+ are given in the table below for 6-coordinate, octahedral geometry [7].
| N3- | O2- | F– | Na+ | Mg2+ | Al3+ | |
| Number of protons | 7 | 8 | 9 | 11 | 12 | 13 |
| Electronic configuration | 2,8 | 2,8 | 2,8 | 2,8 | 2,8 | 2,8 |
| Ionic radius (pm) | 132 | 124 | 119 | 116 | 86 | 67.5 |
The ions are arranged according to their increasing number of protons. Consider the trend among these ions. The ionic radius decreases as the number of protons increases. The increasing number of protons increases the attractive electrostatic force, resulting in smaller radii.
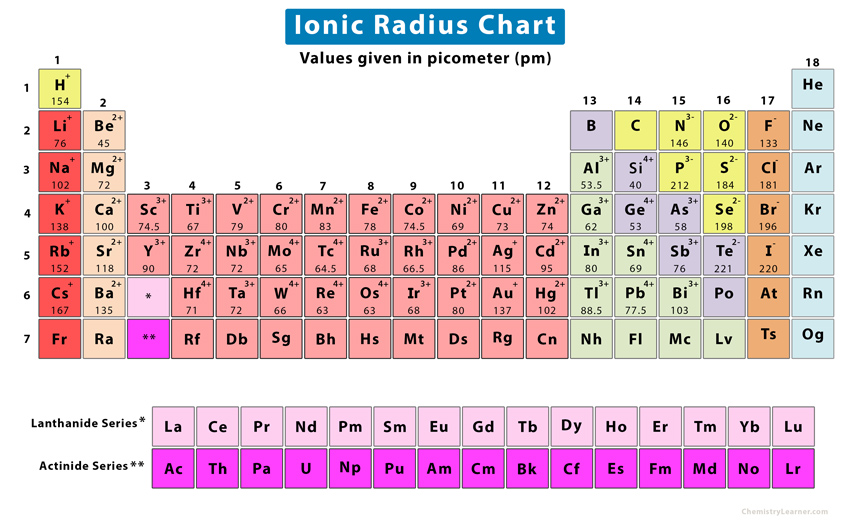
The ionic radius values are shown in the chart below [6].
The ionic radii of d-block elements do not change much. Hence, they do not display a unique periodic trend.