Bredt’s Rule
What is Bredt’s Rule?
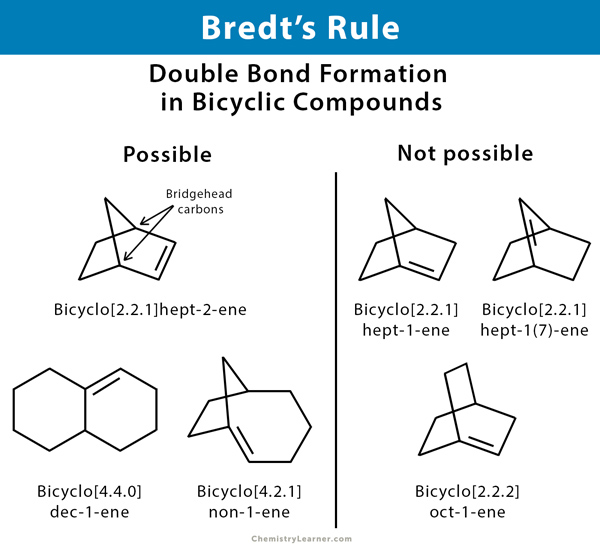
Bredt’s rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system unless one of the rings is big. A bridgehead is an atom that is part of two or more rings in a polycyclic molecule. In a bicyclic compound, there are two bridgehead carbon atoms. Trans cycloalkenes are not stable unless there are at least eight carbon atoms in the ring. Bredt’s rule is valid because the strain on the ring pulls the p-orbitals into different planes, so they cannot line up to form a pi bond.
Brent rule is a consequence of the fact that having a double bond on the bridgehead would be equivalent to having a trans double bond on a ring, which is not possible for rings with fewer than eight atoms. This impossibility is due to a combination of ring strain and angle strain for nonplanar alkene. The p-orbitals of the bridgehead atom and adjacent atoms are orthogonal. Thus, they are not appropriately aligned for the formation of pi bonds [1-5].
The rule is named after Julius Bredt, who first discussed it in 1902 and organized it in 1924.
Application of Bredt’s Rule
Bredt’s rule can be useful for predicting the isomer that is obtained from an elimination reaction in a bridged ring system. It can also be applied to reaction mechanisms that go via carbocations and, to a lesser degree, via free radicals, because these intermediates, like carbon atoms involved in a double bond, prefer to have a planar geometry with 120-degree angles and sp2 hybridization.
References
- Definition – Study.com
- Definition – Onlinelibrary.wiley.com
- Definition – Chem.libretexts.orge
- Definition and example – Chem.ucla.edu
- Definition and example – Pubs.rsc.org