Buffer Solution
What is a Buffer Solution [1-3,5-6]
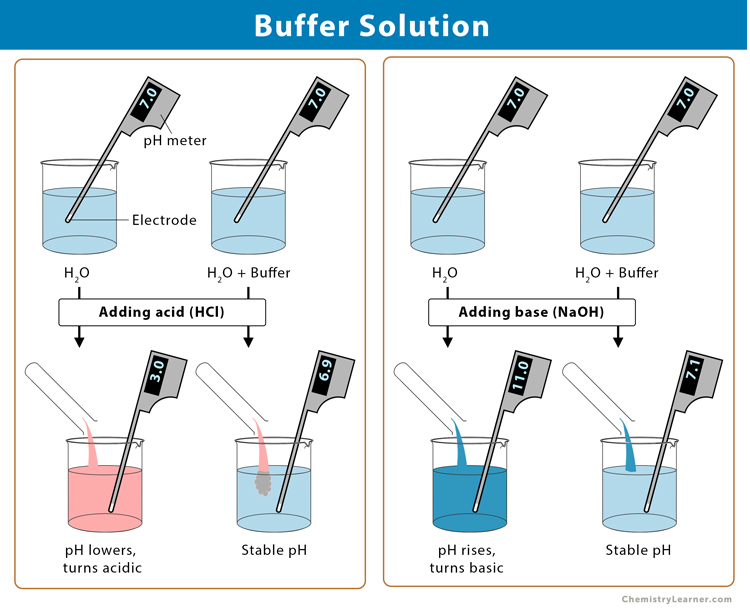
An aqueous solution that resists any change in pH by adding a small amount of acid or base is called a buffer solution. A buffer solution consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
A buffer solution can resist pH change because of an equilibrium between the acid (HA) and its conjugate base (A–). The balanced equation for this reaction is:
HA ⇋ H+ + A–
When a few drops of a strong acid are added to an equilibrium mixture of the weak acid and its conjugate base, the hydrogen ion (H+) concentration increases very little compared to the volume expected for the quantity of strong acid added. It happens because the equilibrium shifts to the left, following Le Chatelier’s principle. If a strong base is added to the equilibrium mixture, the reaction moves to the right to compensate for the lost H+.
As the buffer’s pH changes slightly when a small amount of strong acid or base is added, it is used to prevent any change in the pH of a solution, regardless of solute. Using buffer solutions in various chemical applications keeps the pH at a nearly constant value.
Characteristics of a Buffer Solution
Some of the characteristics of a buffer solution are as follows:
- It has a definite pH
- Its pH remains the same on standing for a long time
- Its pH does not change on dilution
- Its pH changes negligibly by the addition of a small amount of acids or base
Types of Buffer Solution [6]
Primarily, buffer solutions are of two types: acidic and basic buffers.
Acidic Buffers
A buffer solution prepared with large quantities of a weak acid and its salt with a strong base is known as an acid buffer. As the name suggests, these buffer solutions have an acidic pH and are used to maintain acidic environments.
Example
A typical example of an acidic buffer is a mixture of an equal concentration of acetic acid (CH3COOH) and sodium acetate (CH3COONa) [pH = 4.74].
Basic Buffers
A buffer solution containing relatively large quantities of a weak base and its salt with a strong acid is called a basic buffer. It has a basic pH and is used to maintain basic conditions.
Example
A typical example of a basic buffer solution would be a mixture of ammonium hydroxide (NH4OH) and ammonium chloride (NH4Cl) [pH = 9.25].
How to Make a Buffer Solution [2]
There are a few ways to prepare a buffer solution with a specific pH.
In the first approach, a solution with acid and its conjugate base is prepared by dissolving the acid in about 60% of the water required to obtain the final volume of the solution. Next, measure the pH of the solution using a pH probe. Then, using a strong base like NaOH, the pH can be adjusted to the desired value. Alternatively, suppose the buffer is prepared with a base and its conjugate acid. In that case, the pH can be altered using a strong acid like HCl. Once the proper pH is achieved, dilute the solution to the final desired volume.
Alternatively, solutions of both the acid form and base form of the solution can be prepared. Both solutions must have the same buffer concentration as the final buffer solution. To achieve the final buffer, add one solution to the other while monitoring the pH.
In the third method, the exact amount of acid and conjugate base needed to make a buffer of a certain pH can be determined using the Henderson-Hasselbach equation: pH = pKa + log[A−][HA].
How to Calculate pH of Buffer Solution [2-4]
To calculate the pH of an acidic or basic buffer solution, the Henderson-Hasselbalch equation is used.
A weak acid dissociates as follows:
HA(acid) + H2O(water) ⇄ A–(conjugate base) + H3O+(hydronium ion)
The Henderson-Hasselbalch equation is given by:
pH = pKa + log10[A–]/[HA]
Where:
– pH: pH value
– Ka: acid dissociation constant
– [A–]: Concentration of the conjugate base in moles per liter
– [HA]: Concentration of the acid in moles per liter
Similarly, a weak base dissociates as follows:
B + H2O ⇄ OH– + HB+
The Henderson-Hasselbalch equation for a base is given by:
pOH = pKb + log10 ([HB+]/[B])
Where:
– pOH: pOH value
– Kb: base dissociation constant
– [HB+]: Concentration of the conjugate acid in moles per liter
– [B]: Concentration of the base in moles per liter
Factors Determining pH of Buffer Solution
The two factors that determine the pH of the buffer solution are as follows:
- The equilibrium constant (Ka) of the weak acid: The equilibrium constant of a weak acid reveals the proportion of HA that will be dissociated into H+ and A– in solution. If more H+ ions are created, the resulting solution becomes more acidic, thus lowering its pH.
- The ratio of weak acid [HA] to weak base [A–] in solution: If a buffer solution contains more base than acid, more OH– ions are likely to be present, thus raising the pH. On the contrary, if the solution has more acid than base, more H+ ions will be present, thus lowering the pH. When the concentrations of HA and A– are equal, the concentration of H+ becomes equal to Ka (or equivalently pH = pKa).
Mechanism of Buffer Solution [5-6]
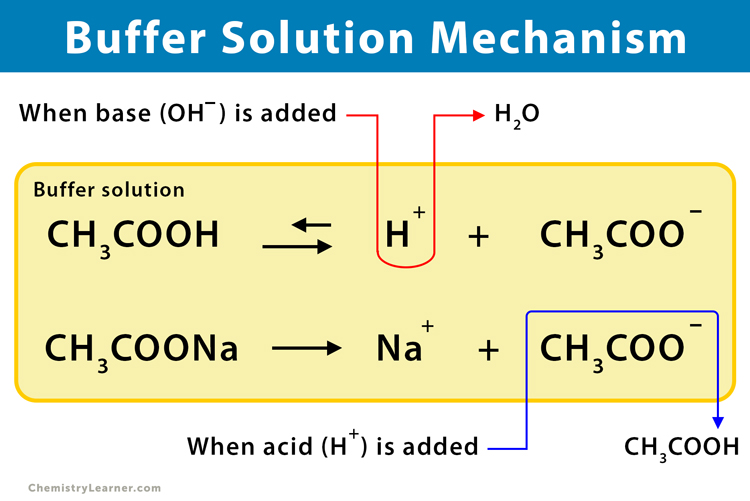
To understand the mechanism of buffer action, let us take an example of an acidic buffer solution of acetic acid (CH3COOH) and sodium acetate (CH3COONa).
In the aqueous medium, they dissociate as:
CH3COONa (aq) ⇌ CH3COO– (aq) + Na+ (aq) [Completely ionised]
CH3COOH (aq) ⇌ CH3COO– (aq) + H+ (aq) [Partially ionised]
When a small quantity of strong acid, like HCl, is added to the buffer solution, the additional hydrogen ions combine with the conjugate base (CH3COO–) as follows:
H+ (aq) + CH3COO– (aq) ↔ CH3COOH (aq)
Since the extra H+ ions get consumed, the pH of the resulting solution remains constant. As CH3COOH is a weak acid and its ions have a strong tendency to form non-ionized CH3COOH molecules, the reaction nearly goes to completion.
On the contrary, if a strong base like NaOH is added, the additional OH– ions get neutralized:
CH3COOH (aq) + OH– (aq) → CH3COO– (aq) + H2O (l)
Also, the added OH– ion reacts with the H+ ion to produce water. As a result, the added OH– ions get removed, and the acid equilibrium shifts to the right to replace the used-up H+ ions. Thus, the pH changes negligibly.
Uses and Applications of Buffer Solution in Daily Life [6]
Buffer solutions have various uses and applications in everyday life. Some of them are as follows:
- Maintenance of Life: As most of the biochemical processes in our bodies work within a relatively narrow pH range, the body uses different buffers, such as carbonate and bicarbonate buffer, to maintain a constant pH close to 7.4.
- Biochemical Assays: As enzymes act on a particular pH, buffer solutions keep the pH value constant during an enzyme assay.
- Shampoos: Buffers such as citric acid and sodium hydroxide are used in shampoos to balance out the alkalinity that would otherwise burn our scalp. These buffers counteract the alkalinity of the detergents present in the shampoo.
- Baby Lotions: Baby lotions are buffered to a pH of about 6 to hinder the growth of bacteria within the diaper and also help prevent diaper rash.
- Brewing Industry: Buffer solutions are added before fermentation begins to make sure the solution is not too acidic and prevent the product from spoiling.
- Textile Industry: Many dyeing processes use buffers to maintain the correct pH for various dyes.
- Laundry Detergents: Many laundry detergents use buffers to prevent their natural ingredients from breaking down.
- Contact Lens: Buffer solutions are used in contact lens solutions to ensure the pH level of the fluid remains compatible with that of the eye.
Solved Problems
P.1. Calculate the pH of the solution containing 0.50M sodium acetate and 0.06M acetic acid. (pKa for CH3COOH = 5.47)
Soln. According to Henderson-Hasselbalch equation, the pH of the acidic buffer is:
pH = pKa + log([Salt]/[Acid])
Putting the values,
pH = 5.47 + log(0.50/0.06)
=> pH = 5.47 + [log(0.50) − log(0.06)]
=> pH = 5.47 + (-0.9208)
=> pH = 5.47 – 0.9208
=> pH = 4.54
P.2. How many moles of sodium acetate and acetic acid each should be dissolved to prepare one liter of 0.063 molar buffer solution of pH 4.5? (Ka for CH3COOH = 1.8×10−5)
Soln. Applying Henderson-Hasselbalch equation:
pH = pKa + log([Salt]/[Acid])
Calculating pKa:
pKa = −log(Ka) = −log(1.8 x 10−5) = 4.74
Therefore:
log([Salt]/[Acid]) = 4.5 – 4.74 = −0.2447
=> [Salt]/[Acid] = 10(−0.2447) = 0.5692
=> [Salt] = 0.5692 [Acid]
From the total concentration:
=> [Salt] + [Acid] = 0.063
=> 0.5692 [Acid]+ [Acid] = 0.063
=> 1.5692 [Acid] = 0.063
=> [Acid] = 0.063/1.5692 = 0.04M
This is the amount of acetic acid.
Now, calculating the amount of sodium acetate:
[Salt] = 0.063 – 0.04 = 0.0228M
FAQ
Ans. As all the reactions between the metal ions and EDTA are pH-dependent, a buffer solution is used in EDTA titration as it resists the change in pH.
Ans. The three major buffer systems of the human body are the carbonic acid bicarbonate buffer system, phosphate buffer system, and protein buffer system.
Ans. To determine the hardness of water, an indicator and EDTA are used. In order to analyze the water, the sample must be kept at a constant pH. As both EDTA and the indicator are weak acids, a buffer solution is used to maintain a reasonably constant pH even when acids and bases are added.
References
- Introduction to Buffers – Chem.libretexts.org
- Buffer Solutions – Courses.lumenlearning.com
- Buffer Solutions – Chem.purdue.edu
- How can we predict the pH of a buffer? – Chemcollective.org
- Mechanism of buffer action – Qsstudy.com
- Buffer solutions – Researchgate.net