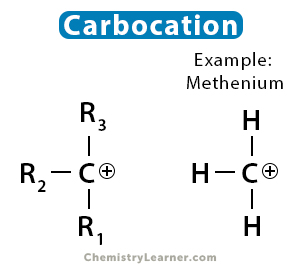
Carbocation
A carbocation is an ion with a positively charged carbon. Before the 1970s, all carbocations were known as carbonium ions [1,4].
Structure and Properties
Carbocations are very reactive species and form an intermediate during electrophilic addition reactions. A distinct feature of the carbocation is that it has six electrons in its outermost electron instead of eight [1,2].
The hybridization of the carbocation is sp2, which means it has three substituents resulting in a trigonal planar molecular geometry. All three substituents are in the same plane, forming an angle 1200 between them. The six outermost electrons are used to create three sigma bonds. A vacant p orbital indicates that it is electron-deficient in nature, a species known as an electrophile. Therefore, it reacts immediately with a nucleophile, like OH– or Cl–, fulfills its octet of electrons, and regains neutral charge.
Types
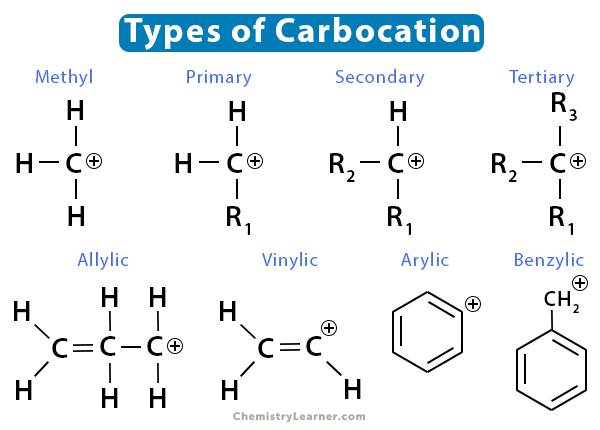
Carbocations are classified based on the number of carbon groups bonded to the positively charged carbon [1,3].
- Methyl – If no carbon group is attached to the positively-charged carbon, it is known as methyl carbocation. Examples are methenium (CH3+) and methanium (CH5+).
- Primary – If one carbon group is attached to the positively-charged carbon, it is known as primary or 10 carbocation. An example is ethanium (C2H7+).
- Secondary – If two carbon groups are attached to the positively-charged carbon, it is known as secondary or 20 carbocation.
- Tertiary – It is known as tertiary or 30 carbocation if three carbon groups are attached to the positively-charged carbon.
- Allylic – If a carbon-carbon double bond is next to the positively-charged carbon, it is termed allylic carbocation—for example, CH2=CH-CH2+.
- Vinylic – If a carbon-carbon double bond is attached to the positively-charged carbon, it is called vinylic carbocation. The hybridization of the carbon atom is sp, and the molecule is linear.
- Arylic – If the positively-charged carbon is part of a benzene ring, it is known as arylic carbocation.
- Benzylic – If the positively-charged carbocation is next to a benzene ring, it is known as benzylic carbocation—for example, C6H5-CH2+.
Carbocation Stability
Carbocation stability is crucial because it determines the rate and mechanism of reactions involving carbocation intermediates. The stability depends on the following factors.
1. Resonance
A carbocation is deficient in electrons. Anything that can donate electrons can stabilize it. Therefore, a carbocation is stabilized by resonance, especially if there is a double or triple bond next to the positively-charged carbon. It has extra stability because of the overlap of the carbocation’s empty p orbital with the p orbitals of the pi bond. This orbital overlap allows the charge to be shared between multiple atoms, a phenomenon known as delocalization. Delocalization of pi electrons stabilizes the carbocation. This is why allylic and benzylic carbocations are more stable than other structures [1].
2. Substitution
Experiments have shown that increasing the number of alkyl substituents increases the stability of the carbocation. Therefore, the most substituted positively-charged carbon will be the most stable. Ranking the carbocation in the order of decreasing stability, we find that [2,4].
Tertiary carbocation > secondary carbocation > primary carbocation > methyl carbocation
Therefore, methenium (CH3+) is the least stable carbocation. As discussed below, the alky group stabilizes a carbocation by two means.
3. Induction and Hyperconjugation
The first reason for stability is because of the inductive effect. The inductive effect involves the drawing of electrons by an atom in a covalent bond toward itself. It generally happens with a highly electronegative atom. Since the positively-charged carbon draws the electrons from its substituents, it gains stability by neutralizing. Alkyl groups can inductively contribute electrons more than hydrogen because they are larger, more polarizable, and contain more bonding electrons. So, the more alkyl substitutions, the more stable the carbocation [2,3].
The second reason for stability is because of hyperconjugation. Hyperconjugation involves donating electrons from the parallel overlap of p orbitals with adjacent hybridized orbitals from sigma bonds. This process stabilizes the carbocation. Increasing the number of alkyl substituents increases the number of sigma bonds available for hyperconjugation. Therefore, the carbocation becomes more stabilized.
Just as an electron-donating group can stabilize a carbocation, an electron-withdrawing group, like carbonyl (-C=O), can destabilize a carbocation through the same inductive effect.