Ionic, Covalent, and Metallic Bonds
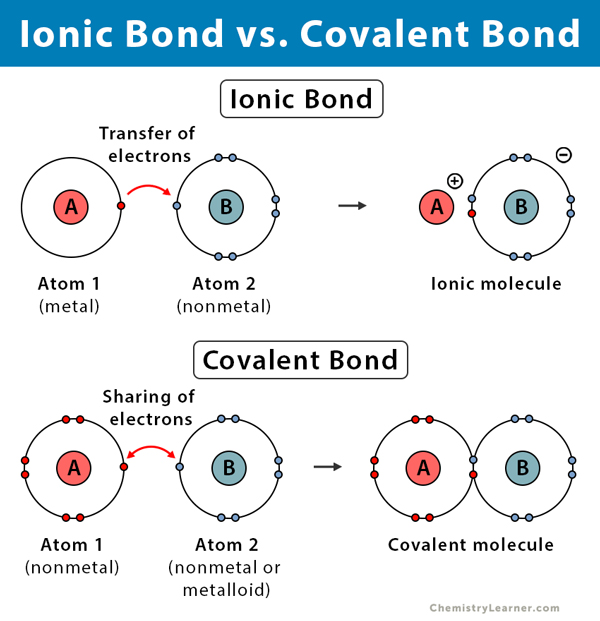
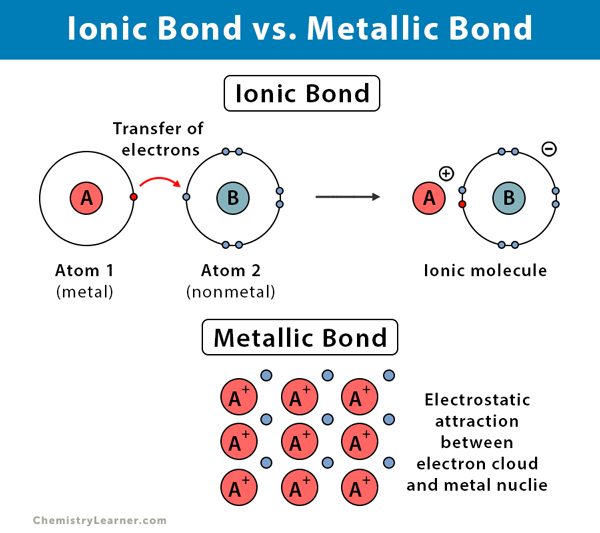
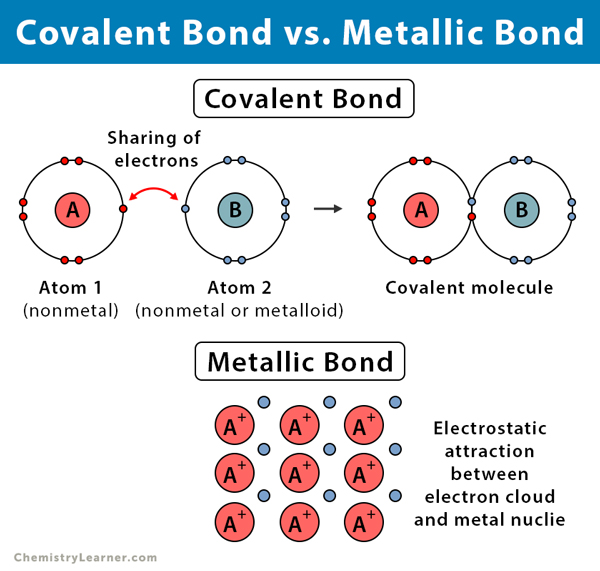
Ionic, covalent, and metallic bonds are different types of chemical bonds. An ionic bond is formed when one atom donates valence electrons to another atom. A covalent bond is formed when both atoms share pairs of valence electrons. A metallic bond is formed between a cloud of free electrons and the positively charged ions in a metal.
In ionic and covalent bonds, the valence electrons play a critical role in forming the bond. Atoms achieve a stable electronic configuration by transferring and sharing electrons. As a result, the bonds become stable with well-defined strength and energy.
Ionic Bond vs. Covalent Bond vs. Metallic Bond | |||
| Ionic Bond | Covalent Bond | Metallic Bond | |
|---|---|---|---|
Occurs between | A metal and a nonmetal | Two nonmetals or a nonmetal and a metalloid | Positively charged ions and negatively charged electron cloud |
Formation | Electrostatic attraction between oppositely charged ions | Sharing pairs of electrons | Electrostatic attraction between the delocalized electron cloud and positively charged metal ions |
Formation between atoms of the same element | No | Yes | – |
Electronegativity difference between atoms | High (>2) | Low (<0.1) for nonpolar compounds and intermediate (0.1 – 2) for polar compounds | Electronegativity does not play any role |
Isomerism | Nondirectional | Directional | Nondirectional |
Physical state of compounds | Solid at room temperature | Liquid or gas at room temperature | Solid at room temperature |
Physical properties | High melting and boiling points | Low melting and boiling points | High melting and boiling points |
Dissociate into ions in solution | Retain their molecular identity in solution | Some metals react vigorously with water, while others do not | |
Conductivity | Low in solid-state, but becomes high in molted state and solutions | Low, except conducting polymers | High |
Examples | Sodium chloride (NaCl) and potassium iodide (KI) | Methane (CH4) and water (H2O) | Sodium (Na) and potassium (K) |
Similarities between Ionic and Covalent Bonds
- Valence electrons participate in bonding
- Form neutral, stable compounds
- Compounds are formed through exothermic reactions
- Compounds have faster rates of reactivity
- Ionic compounds are always polar. Some covalent compounds are also polar.
- Ionic compounds are crystalline. Some covalent compounds are also crystalline.
Similarities between Ionic and Metallic Bonds
- Involve electrostatic attractions
- Metallic bond has high thermal and electrical conductivities. The ionic bond can have high conductivities in molten states and solutions.
- Ionic compounds and metals have high melting and boiling points.
- Ionic compounds and metals are solid at room temperature
Similarities between Covalent and Metallic Bonds
- Metals are solid at room temperature. Some covalent compounds are solid at room temperature.