Solubility
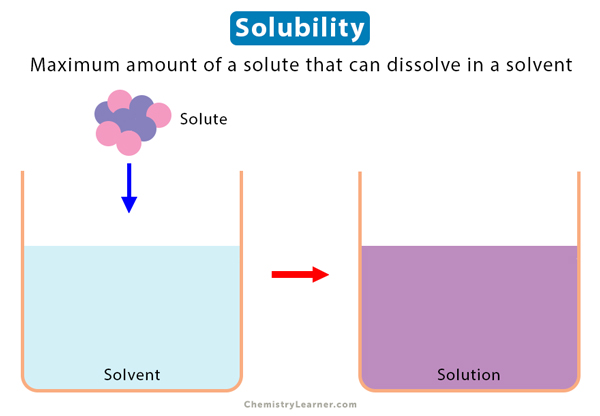
When you put a spoon of table salt (solute) in water (solvent) and stir it, the salt disappears. It is because salt dissolves and forms a saline solution. There is a limit to the amount of salt that can dissolve in water. Solubility is the maximum concentration of a solute that can dissolve in a specific amount of a solvent at a given temperature. The process through which a solute in its solid, liquid, or gaseous phase dissolves in a solvent to produce a solution is called dissolution. Thus, solubility is a consequence of dissolution [1-4].
Solubility is a physical property, not a chemical property. The reason is that solubility is measured by making practical observations and not by altering the chemical composition of the solute or solvent or both. In the example above, the composition of salt and water does not change during the dissolving process. Also, solubility is an intensive property, which means that it does not depend on the quantity of the solute.
Water is the most widely used solvent due to its excellent properties. It is a polar compound capable of forming hydrogen bonds and dissolving several molecules. Solubility is measured in units of grams per liter (g/L). For example, the solubility of table salt in water at 25 ˚C is 360 g/L. When the solubility is measured in moles per liter (mol/L), it is called molar solubility.
Examples of Solubility [1-6]
Sodium Chloride (NaCl)
Let us go back to the example of salt and water. Table salt or common salt is sodium chloride (NaCl). NaCl dissolves in water; it breaks down into sodium ions (Na+) and chloride ions (Cl–). The dissolving reaction is shown as follows:
NaCl (s) ⇌ Na+ (aq.) + Cl– (aq.)
Here, the dissolution of NaCl is the forward reaction, and the combination of Na+ and Cl– ions to form NaCl is the reverse reaction. The two reactions compete with each other until they reach an equilibrium. At this point, NaCl has reached its maximum concentration, and no more salt can dissolve. When the solute’s concentration reaches its solubility, the solution is said to be saturated with the solute. If the solute’s concentration is less than its solubility, the solution is said to be unsaturated.
Other Examples
1. Solid in Liquid
A. Sugar consists of sucrose (C12H22O11). When sugar dissolves in water, the delicate bonds between the individual molecules are broken and released into the solution. The slightly polar sucrose molecules form intermolecular bonds with the polar water molecules while releasing energy.
C12H22O11 (s) ⇌ C12H22O11 (aq.)
B. The principle of an instant cold pack is based on solubility. A cold pack consists of ammonium nitrate (NH4NO3) and water (H2O) in separate bags, one inside the other. When you squeeze the pack, the inner bag breaks and mixes the two substances. The dissolving reaction is endothermic and takes heat from the body, making you feel cold.
NH4NO3 (s) → NH4+ (aq.) + NO3– (aq.)
2. Gas in Liquid
A. Many gases dissolve in water.
- Ammonia – Forms an alkaline solution
- Oxygen – Supports aquatic life
- Hydrogen chloride – Forms hydrochloric acid solution
- Carbon dioxide – Slightly dissolves to form weak carbonic acid
- Chlorine – Forms chlorinated water
B. A well-known example of a gas dissolving in liquid is carbon dioxide in a beverage. This process is known as carbonation and is the basis for manufacturing carbonated drinks. It involves exposing the beverage to pressurized carbon dioxide and then sealing the beverage bottle, thus saturating the beverage with the gas. When the bottle is opened, a hissing sound is heard due to the pressurized gas being released. Some dissolved carbon dioxide leaves the solution as tiny air bubbles.
3. Liquid in Liquid
A. Liquids mix in all proportions to form homogenous solutions, a property known as miscibility. In other words, some liquids have infinite solubility. Such liquids are said to be completely miscible. An example of a completely miscible liquid is ethylene glycol in water, popularly known as antifreeze. Other water-soluble liquids include ethanol and sulfuric acid. Generally, polar liquids mix with other polar liquids and nonpolar liquids mix with other nonpolar liquids (“like dissolves like”).
B. Liquids can also mix partially. An example of this is bromine and water. The two are said to be partially miscible. When water and bromine are mixed, two very distinct layers are formed on top of one another. The lighter-colored upper layer is a saturated solution of bromine in water. The darker-colored lower layer is a saturated solution of water in bromine.
Factors Affecting Solubility
Temperature and pressure are two main factors affecting the solubility of solids, liquids, and gases [1-6].
Temperature
1. Gas
For gas, the solubility decreases with temperature. The reason is that when most gases dissolve in solvents, the process is exothermic. Heat is released, and the temperature rises. Increasing temperature increases kinetic energy, resulting in more vigorous motion of the gas molecules. Eventually, the intermolecular bonds break, and the gas escapes from the solution.
2. Solid or Liquid
The solubility of a solid or liquid depends on the enthalpy change (ΔH). Whether the dissolving process is endothermic (ΔH > 0) or exothermic (ΔH < 0), the solubility may increase or decrease with temperature.
Case I: Decrease in solubility with temperature.
Suppose the heat given off during dissolving is greater than the heat required to break apart the bonds in the solute. In that case, the net dissolving reaction is exothermic. The addition of more heat increases the temperature and impedes the dissolving reaction. The reason is that the reaction is already producing that excess heat. This kind of situation is not very commonly observed.
Case II: Increase in solubility with temperature.
Suppose the heat given off during dissolving is less than the heat required to break apart the bonds in the solute. In that case, the net dissolving reaction is endothermic. The addition of more heat increases the temperature and facilitates the dissolving reaction. The reason is that heat provides the energy necessary to break bonds in the solid. This case is most commonly observed.
Solubility and Le Chatelier’s Principle
Le Chatelier’s Principle can explain the effect of temperature on solubility. According to this principle, if stress, like temperature, pressure, and reactant concentration, is applied to a system at equilibrium, the system will adjust to minimize the stress. This principle is helpful since it can predict how a process will respond to changes in external conditions.
Consider the case in which the solubility process is endothermic. The reaction takes heat from the surroundings and reaches equilibrium during dissolving. Now, suppose heat is added to the process such that the temperature rises and stresses the equilibrium. According to Le Chatelier’s principle, the stress is relieved because the dissolving process uses up some heat, causing the equilibrium to shift towards the right. Therefore, the solubility increases with an increase in temperature.
If the process is exothermic, it gives off heat and raises the temperature. So, if heat is supplied to the process, it will not be utilized for dissolving. Instead, the temperature rise will decrease the solubility by shifting the equilibrium to the left.
Solubility curves are used to plot the relationship between solubility and temperature. They can be used to compare the solubilities of two or more substances.
2. Pressure
Solid and liquid do not show any change in solubility with a change in pressure. However, pressure has the greatest effect on the solubility of a gas in a liquid.
Consider oxygen (O2) gas that dissolves in water. The gas atop the solution is in equilibrium with the dissolved part. The equilibrium reaction is shown as follows:
O2 (g) ⇌ O2 (aq.)
The equilibrium constant (K) can be defined as follows:
K = c (O2)/p (O2)
The expression for equilibrium constant tells that a gaseous solute’s concentration (c) in a solution is directly proportional to its partial pressure (p) above the solution. Increasing the pressure of a gas increases its solubility. This statement is known as Henry’s law.