Acid-Base Reaction
- What is an Acid-Base Reaction?
- Strong Acid and Strong Base Reaction
- How to Identify the Acid and Base in a Reaction
- Predicting Products of Acid-Base Reaction
- Balancing Acid-Base Reaction
- Examples of Acid-Base Reaction
- Acid-Base Reaction in Organic Chemistry
- Reaction Between Weak Acid and Weak Base
- Uses of Acid-Base Reaction in Daily Life
- FAQs
What is an Acid-Base Reaction?
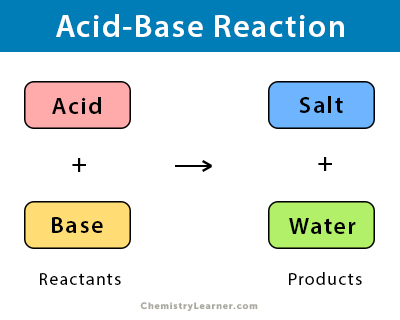
An acid-base reaction is a chemical reaction that occurs between an acid and a base that are the reactants. The products of this reaction are salt and water. An acid-base reaction is a double-replacement reaction where ions exchange their positions. This type of reaction is essential in both biochemistry and industrial chemistry [1,2].
General Equation
The general equation of an acid-base reaction is as follows.
Acid + Base → Salt + Water
Strong Acid and Strong Base Reaction
A strong acid is one that readily gives off hydronium (H+) ions when dissolved in water (H2O). A strong base is one that quickly gives off hydroxyl (OH–) ions when dissolved in water. The H+ and OH– combine to form H2O. Strong acid and base react completely and neutralize each other. Hence, the acid-base reaction is also called neutralization reaction.
How to Identify the Acid and Base in a Reaction
To determine whether a substance is an acid or base, count the hydrogens before and after the reaction. If the number of hydrogens has decreased, that substance is the acid because it donates hydrogen ions. If the number of hydrogens has increased, that substance is the base since it accepts hydrogen ions.
Predicting Products of Acid-Base Reaction
In most acid-base reactions, there will always be water and salts. For example, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) salt and water (H2O).
HCl (aq.) + NaOH (aq.) → NaCl (aq.) + H2O (l)
Balancing Acid-Base Reaction
To balance a reaction, we must make sure that there is an equal number of atoms of a particular element on both sides of the equation. Consider the example of the reaction between phosphoric acid (H3PO4) and calcium hydroxide Ca(OH)2 resulting in insoluble Ca3(PO4)2 and water (H2O).
H3PO4 (aq.) + Ca(OH)2 (aq.) → Ca3(PO4)2 (s) + H2O (l)
This equation is unbalanced. We need two phosphates (PO4+) and three calcium (Ca+) ions on the left. So, we multiply the reactants accordingly and obtain:
2 H3PO4 (aq.) + 3 Ca(OH)2 (aq.) → Ca3(PO4)2 (s) + H2O (l)
No, we find that there are six O atoms in 3 Ca(OH)2. So, we multiply H2O by 6, thereby balancing both O and H.
2 H3PO4 (aq.) + 3 Ca(OH)2 (aq.) → Ca3(PO4)2 (s) + 6 H2O (l)
The chemical equation is now balanced.
Examples of Acid-Base Reaction
Most acid-base reactions occur between strong acids and bases, resulting in complete neutralization. Below are some examples, along with the formula of the compounds [1-4].
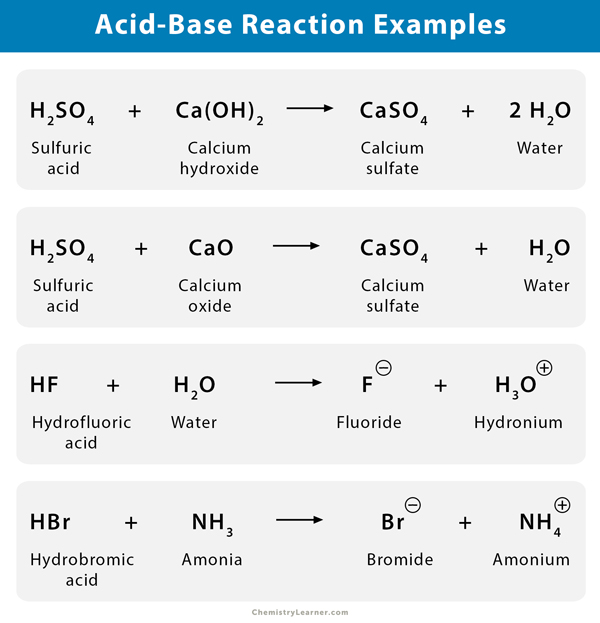
- Sulfuric acid (H2SO4) and potassium hydroxide (KOH) react to form potassium sulfate (K2SO4) and water (H2O).
H2SO4 (aq.) + 2 KOH (aq.) → K2SO4 (aq.) + 2 H2O (l)
- Ammonia (NH3) dissolves in hydrofluoric acid (HF) to form ammonium salt (NH4–) and fluoride (F–).
HF (aq.) + NH3 (aq.) → NH4+ (aq.) + F– (aq.)
- Hydrogen bromide (HBr) reacts with magnesium hydroxide (Mg(OH)2), and the products are magnesium bromide (MgBr2) and water (H2O).
Mg(OH)2 (aq.) + 2 HBr (aq.) → MgBr2 (aq.) + 2 H2O (l)
- Ammonia (NH3) and hydrogen sulfate (H2SO4) reacts to form ammonium sulfate ((NH4)2SO4) salt.
H2SO4 (aq.) + 2 NH3 (aq.) → (NH4)2SO4 (aq.)
Acid-Base Reaction in Organic Chemistry
Acid-base reactions are also observed in organic chemistry. According to Brønsted-Lowry theory, a compound that donates a proton is an acid, and a compound that accepts a proton is a base [5,6].
Example
- Methanol (CH3OH) reacts with sodium hydride (NaH), producing the sodium salt of methanol (CH3O–Na+) and hydrogen gas (H2).
CH3OH (aq.) + NaH (aq.) → CH3O–Na+ (aq.) + H2 (g)
Reaction Between Weak Acid and Weak Base
Complete neutralization does not occur when a weak acid reacts with an equivalent amount of a weak base. The concentrations of the species in equilibrium with each other will depend on the equilibrium constant, Ka , for the reaction. The general equation can be represented as:
AH + B ⇌ A– +BH+
Example
- Acetic acid (CH3COOH) gives off proton (H+) atom to water (H2O), resulting in acetate (CH3COO–) ion and hydronium (H3O+).
CH3COOH (aq.) + H2O (l) ⇌ CH3COO– (aq.) + H3O+ (aq.)
Uses of Acid-Base Reaction in Daily Life
Acid-base reactions are significant in everyday life.
- Calcium oxide (CaO) is used to neutralize acidic soil.
- Hydrochloric acid (HCl) in the stomach plays a vital role in digesting food. Antacids, which are bases, are taken to neutralize excess stomach acid, to prevent damage to the intestines.
- Alkaline calcium hydroxide (limewater) is used to absorb harmful acidic sulfur dioxide (SO2) gas that is released from power stations and the burning of fossil fuels.
FAQs
Ans. Increasing the temperature will cause particles to collide with more energy so they will have sufficient activation energy to trigger a reaction. Reducing the temperature will have the opposite effect.
Ans. Acid is used in food to slow down or prevent the growth of disease or spoilage, causing organisms. This reaction can prolong the shelf life of food while allowing the nutrient value to remain relatively unchanged.