Collins Reagent
Definition: What is Collins Reagent?
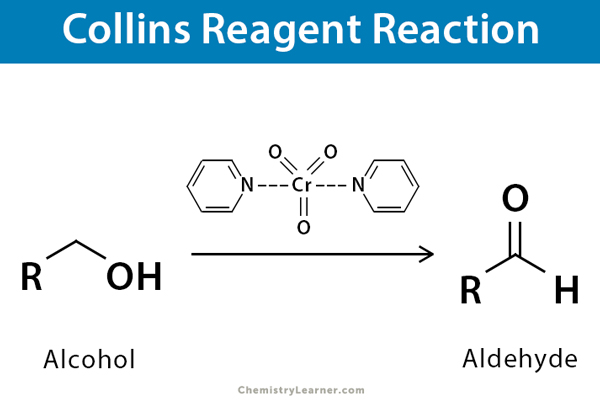
Collins reagent is the complex of chromium (VI) oxide with pyridine in dichloromethane. It is a red-colored metal-pyridine complex and is used to oxidize primary alcohols to aldehyde without overoxidation. Its molecular formula is C10H10CrN2O3. It is useful for oxidizing acid-sensitive compounds, and the process is known as Collins oxidation. Collins reagent can be used as an alternative to the Jones reagent, Sarett Reagent, and pyridinium chlorochromate (PCC) when oxidizing secondary alcohols to ketones [1].
The reagent is named after Joseph C. Collins, who reported it in two publications in 1968 and 1972.
Preparation of Collins Reagent
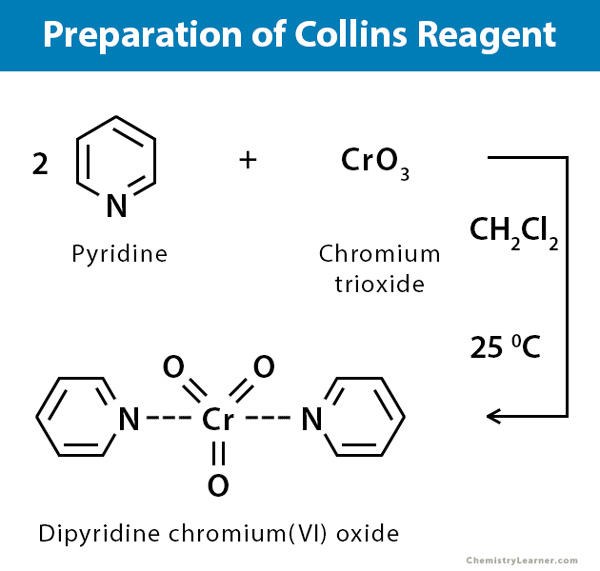
Collins reagent or dipyridine chromium (VI) oxide is prepared by the addition of one equivalent chromium trioxide to a stirred solution of two equivalents of pyridine in methylene. It precipitates in a yellow microcrystalline form, and on continuous stirring at 15°C, it reverts to a deep red macrocrystalline form, which can be isolated, dried, and stored. This complex is both challenging and dangerous to make. The reaction of chromium trioxide with pyridine is very hygroscopic, exothermic, and can inflame during its preparation. The preparation should be conducted in a hood, observing the precautions [2-4].
Example of Collins Reagent Reaction
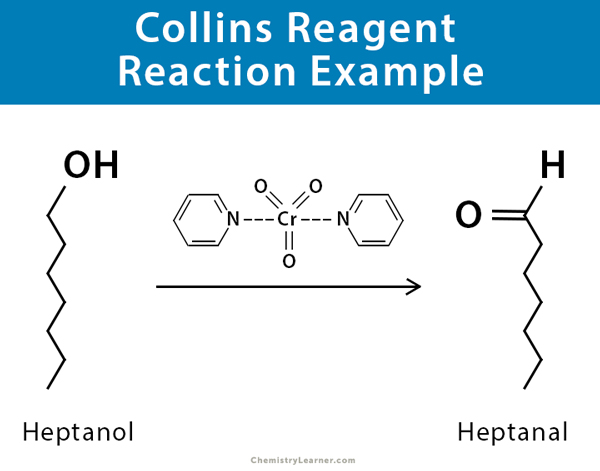
Collins reagent can be used to convert heptanol to heptanal [5,6].
Mechanism of Collins Oxidation Reaction
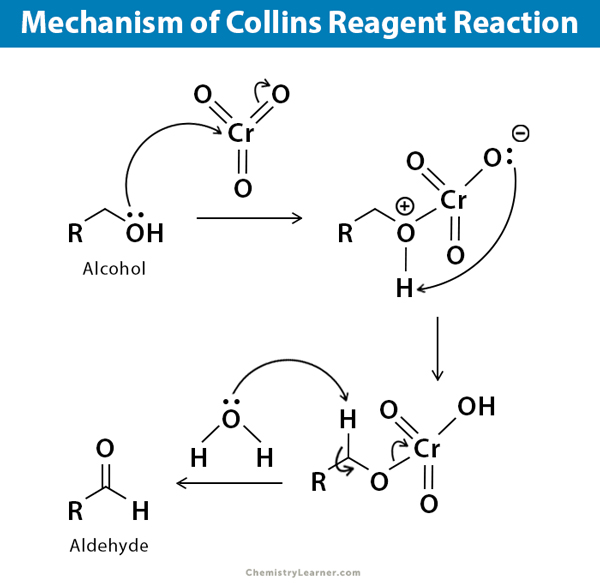
The reaction of Collins reagent with alcohol is sometimes called Collins reagent oxidation reaction. The mechanism is a short and straightforward oxidation process [7].
References
- Definition – Nptel.ac.in
- Preparation – Shodhganga.inflibnet.ac.in
- Preparation – Onlinelibrary.wiley.com
- Preparation – Sciencedirect.com
- Example – Orgsyn.org
- Example – Vanderbilt.edu
- Mechanism – Chem.libretexts.org