Galvanic Cell (Voltaic Cell)
A galvanic cell, also known as a voltaic cell, is a device that can convert chemical energy into electrical energy through spontaneous redox (oxidation-reduction) reactions. It is a type of electrochemical cell that is named after Italian scientists Luigi Galvani and Alessandro Volta. They performed their pioneering works in the late 18th century [1-4].
How Does a Galvanic Cell Work
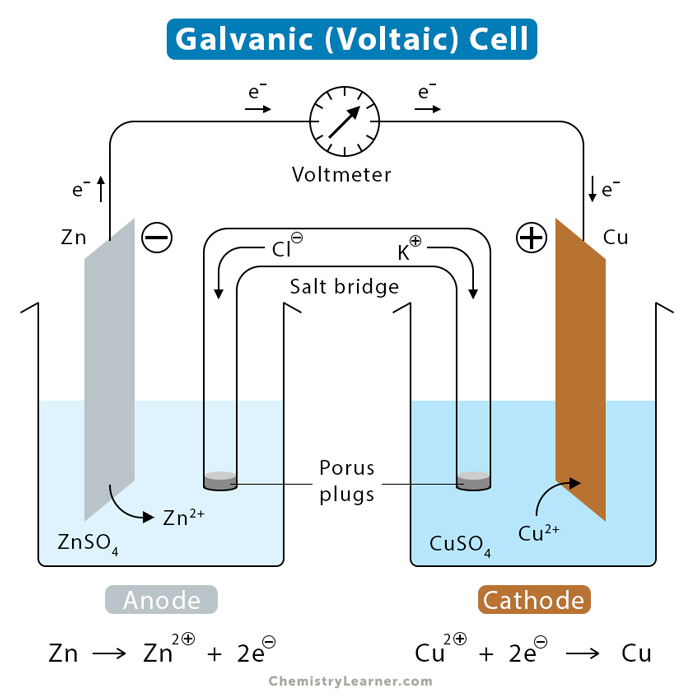
A galvanic cell consists of two separate components, known as half-cells. Each half-cell consists of a metallic electrode dipped into an electrolyte. These two electrodes are connected by a metallic wire that passes through a voltmeter and a switch. While oxidation occurs in one half-cell, reduction occurs in the other [1-5].
The reactions occurring in the two half-cells are called half-reactions, where one metal loses electrons (oxidation) and the other gains electrons (reduction). The electrode where oxidation occurs is the anode, which becomes positively charged. The electrode where reduction occurs is the cathode, which becomes negatively charged. The reducing agent is the reactant that is oxidized. The oxidizing agent is the reactant that is reduced.
Salt Bridge
Because electrons flow from one half-cell to another, they do not complete the circuit. As a result, charge imbalance builds up. The charge build-up is avoided by connecting the two half-cell compartments by a salt bridge. A salt bridge is a U-shaped tube containing a concentrated, nonreactive electrolyte solution of some ionic compound (potassium chloride or ammonium nitrate). The ions of this neutral compound migrate to either side of the bridge to maintain the balance.
Example
To analyze a galvanic cell, let us take the example of a Daniell cell. A Daniell cell consists of two electrodes of zinc and copper dipped in their respective sulfate solutions. The left half-cell is the zinc (Zn) electrode in a zinc sulfate (ZnSO4) solution. The right half-cell is the copper (Cu) electrode in a copper (II) sulfate (CuSO4) solution. Several electrochemical processes occur in a galvanic cell and are discussed below [1-5].
Chemical Equations
Anode
Zinc ions from the zinc electrode are oxidized to zinc atoms. It is because zinc lies higher than copper in the activities series chart and can easily be oxidized by the oxidizing agent, which is the anode. The chemical reaction occurring at the anode is shown below [1-5].
Zn (s) → Zn2+ (aq.) + 2e–
The anode degrades over time due to the loss of zinc. The concentrations of zinc ion and electron increase in this half-cell. The electrons travel through the wire and reach the copper electrode.
Cathode
The electrons from the anode combine with the copper (II) ions in the solution and convert them to copper atoms. The chemical reaction is shown below.
Cu2+ (aq.) + 2e– → Cu (s)
The cathode increases in mass because of the deposition of copper atoms. The concentration of electrons decreases in this half-cell.
The following balanced equation can summarize the two half-reactions.
Zn (s) + Cu2+ (aq.) → Zn2+ (aq.) + Cu (s)
Cell Notation
Since there are many galvanic cells, it is generally appropriate to represent them in shorthand notation. This notation shows the cell components from left to right [1-5].
Anode|Anode Electrolyte||Cathode|Cathode Electrolyte
The anode is depicted on the left, and the cathode is on the right. Vertical lines are also included in this notation. A single vertical line indicates the phase boundary, and a double vertical line represents the salt bridge. Based on this notation, the Daniell cell is represented as follows.
Zn|Zn2+(aq., 1 M)||Cu2+(aq., 1 M)|Cu
Here, zinc is on the left and copper on the right. The solid vertical line on the left is the phase boundary between Zn and Zn2+and the same on the right is the phase boundary between Cu and Cu2+. The double vertical line is the salt bridge. One mole of the aqueous solution is written in parenthesis to show that the two half-reactions occur at standard conditions.
Cell Potential
A voltmeter connected across the circuit records the potential difference between the two half-cells. This potential difference is also known as cell potential or electromotive force (emf), represented by the symbol E. The cell potential is created when two dissimilar metals are coupled. It measures the energy per unit charge available from the oxidation-reduction reaction. The standard cell potential Eocell is calculated by the following equation [5].
Eocell = Eocathode – Eoanode
The Eocathode and Eoanode are the standard reduction potential at the cathode and anode, respectively. Their values are tabulated for 1 mole of the solute. The anode has a negative potential with respect to the electrolyte, while the cathode has a positive potential. The Eocell value is positive for a galvanic cell, meaning that the reaction occurring inside the cell is spontaneous. For a Daniell cell, the cell potential is: Eocell = +0.34 – (–0.76) = 1.10 V.
Galvanic Cell vs. Battery
A battery is a device that consists of a series of galvanic cells. It has all the reactants necessary to convert chemical energy into electrical energy. A commercial battery uses solids or pastes instead of a solution to maximize efficiency. Some examples of a battery include Leclanché dry cell, button battery, and lithium-iodine battery.
FAQs
Ans. Increasing the concentration of reactants will increase the voltage. The reason is that a higher reactant concentration allows the reaction in the forward direction. So it reacts faster, resulting in higher voltage.
- Flexbooks.ck12.org
- Courses.lumenlearning.com
- Hyperphysics.phy-astr.gsu.edu
- Opentextbc.ca
- Chem.libretexts.org