Gilman Reagent
Definition: What is Gilman Reagent?
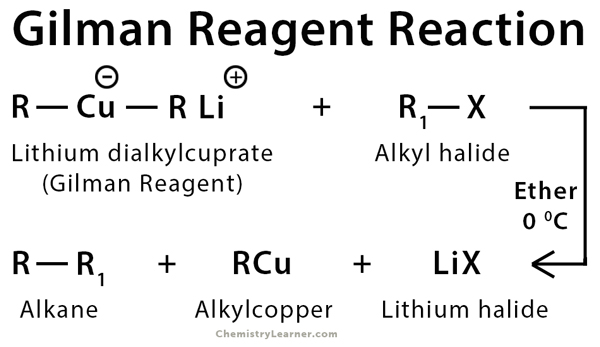
Gilman reagent is an organocuprate reagent consisting of lithium, copper, and alkyl group with the molecular formula [R-Cu-R]+Li– (lithium dialkylcuprate). It is used to synthesize new compounds consisting of carbon-carbon bonds from alkyl, aryl, and vinyl halides. Typically, the SN2 reaction method is employed to obtain the final product. The SN2 reaction mechanism is discussed in another article [1-7].
The history of Gilman reagent goes back to the 1930s when American chemist Henry Gilman discovered it and reported it in a 1952 paper.
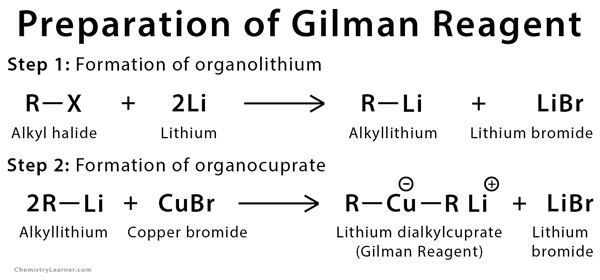
Preparation of Gilman Reagent
Gilman reagent can be prepared in two steps: First, by adding powdered lithium metal to alkyl halide in pentane solvent. Second, by adding copper(I) bromide to alkyllithium in tetrahydrofuran at −78 °C.
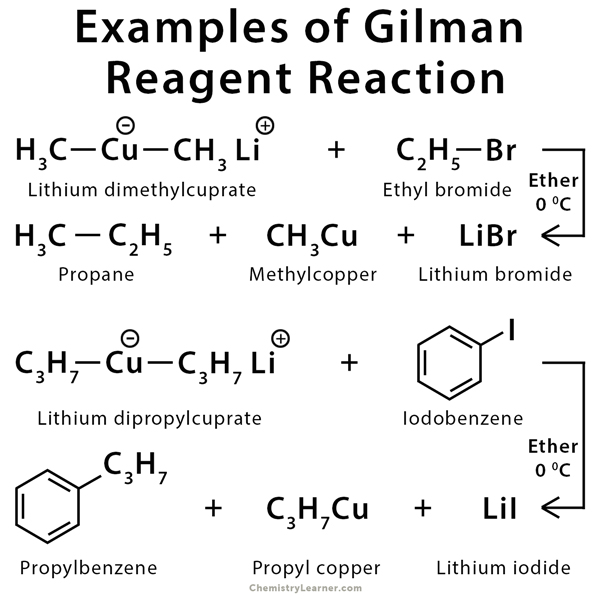
Example of Gilman Reagent Reaction
Gilman reagent is used to synthesize new compounds using SN2 reaction as well as α, β – addition [1-3].
References
- Definition and examples – Testbook.com
- Definition and examples – Chem.libretexts.org
- Definition and example – Nptel.ac.in
- Definition – Chemistry.msu.edu
- Definition – Chem.ucla.edu
- Definition – Organicreactions.org
- Definition – Evans.rc.fas.harvard.edu