Hund’s Rule
What is Hund’s Rule[1-4]
An atom consists of a nucleus around which electrons revolve in well-defined orbits. The electrons reside in orbitals of sublevels of different energy. According to the Aufbau principle, electrons fill the lowest energy sublevel before filling up the higher ones. Thus, electrons are found in discrete atomic sublevels in an arrangement known as electron configuration. However, the filling up of orbitals in the sublevels follows a particular set of guidelines known as Hund’s rule, which states that:
- Every orbital is singly occupied before it is doubly occupied.
- All electrons in singly occupied orbitals spin in the same direction to maximize it.
The rule is named after German physicist Friedrich Hund, who formulated it around 1927.
Why Hund’s Rule is called the Rule of Maximum Multiplicity[2,3]
According to Hund’s rule, the lowest energy term in a given electronic configuration has the highest value of spin multiplicity. The electrons enter the sublevel orbitals unpaired to occupy in maximum number. All of them have identical directions of spins. This electron configuration is known as maximum multiplicity.
Hund’s Rule and Electron Configuration[1-3]
Electron configuration can predict the stability of an atom. If the valence orbitals are not filled, the atom will be unstable and combine with another unstable atom to form chemical bonds. The atom achieves a stable configuration when all the orbitals are filled. Such atoms do not have any empty orbital or unpaired electrons.
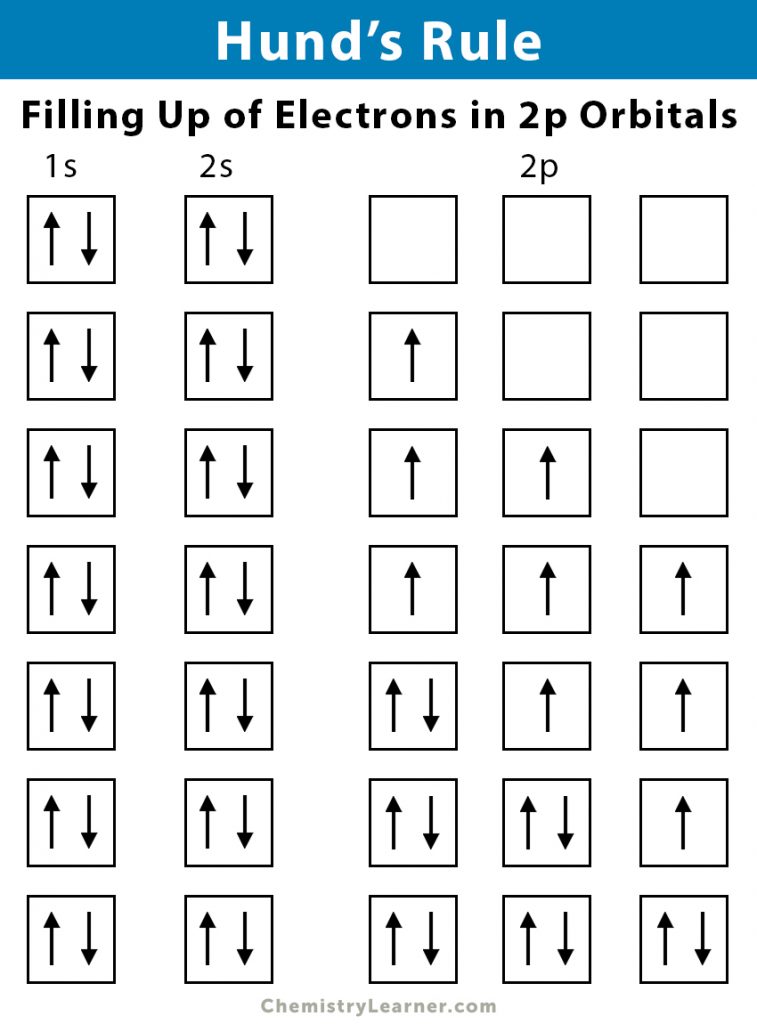
Examples of Hund’s Rule[1,3]
The different sublevels are designated as s, p, d, and f. The maximum number of electrons they can take is as follows:
s-sublevel – 2 electrons
p-sublevel – 6 electrons
d-sublevel – 10 electrons
f-sublevel – 14 electrons
Each sublevel is divided into orbitals, and each orbital can take a maximum of two electrons. The number of orbitals is as follows:
s-sublevel – 1 orbital
p-sublevel – 3 orbitals
d-sublevel – 5 orbitals
f-sublevel – 7 orbitals
Each orbital is initially filled with one electron when the electrons fill them. All the unpaired electrons have the same spin. Then, a second electron with an opposite spin completes the occupancy.
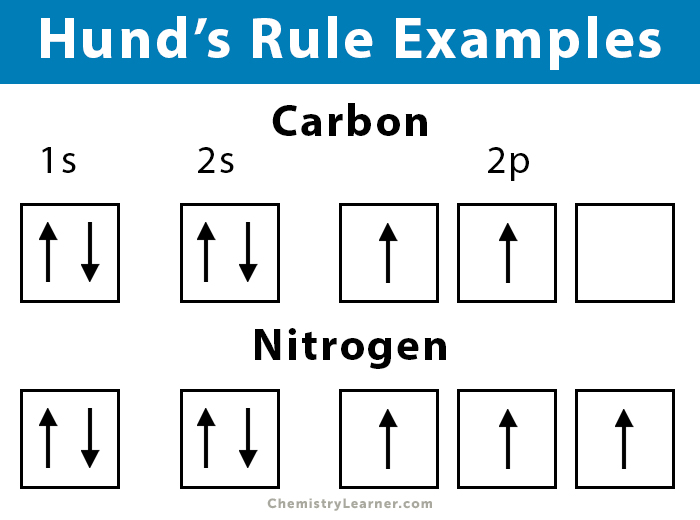
For example, the electron configuration for a carbon atom is 1s22s22p2. According to this configuration, there will be two electrons in each of the 1s and 2s sublevels and 2 electrons in the 2p sublevel. The 1s and 2s sublevels are filled completely, and the 2p sublevel is filled partially. According to Hund’s rule, these two electrons will occupy separate orbitals with the same spin. If they are found in the same orbital, then it will result in a violation of Hund’s rule.
Examples of elements having completely filled subshells are helium, neon, argon, and xenon.
Hund’s Rule and Pauli Exclusion Principle[3]
Pauli exclusion principle states that no two electrons can have the same set of four quantum numbers in a single atom. These quantum numbers are designated by n, l, ml, and ms. Since orbital consists of two electrons, they will only differ in their spin quantum number ms. One electron will have an up spin (ms = +1/2), and the other electron will have a down spin (ms = -1/2). In other words, every electron has a unique (single) state.
Applications of Hund’s Rule[5]
Hund’s rule has a wide application in chemistry, especially in analytical chemistry, spectroscopy, and quantum chemistry.




You’re site is very beautiful and your content is very good as well. Thank you.