Knoevenagel Condensation
Definition: What is Knoevenagel Condensation?
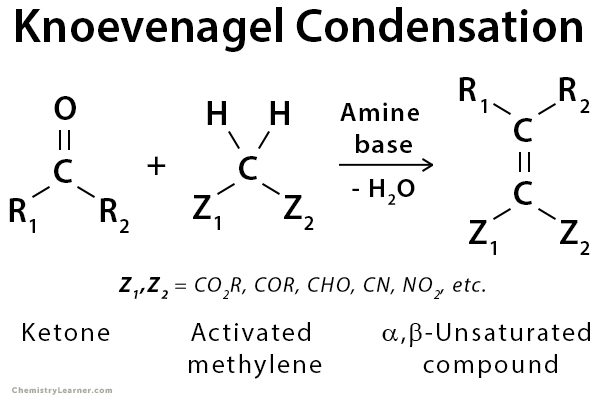
Knoevenagel condensation is a nucleophilic addition of an activated methylene compound to an aldehyde or ketone using an amine base (e.g., pyridine or piperidine) as a catalyst followed by a dehydration reaction in which a molecule of water is eliminated (condensation). The product is often an α,β-unsaturated carbonyl compound (enone). The Knoevenagel reaction is a particular variant of the aldol reaction with subsequent elimination of water. It is widely used in organic chemistry for C=C bond formation. The activated methylene compound should be of the form Z-CH2-Z, where Z is an electron-withdrawing group. Some examples are diethyl malonate (C2H5O-CO-CH2-CO-OC2H5) and malonic acid (HOOC-CH2-COOH) [1-5].
The history of this reaction goes back to 1898, when it was reported by German chemist Emil Knoevenagel.
Examples of Knoevenagel Condensation
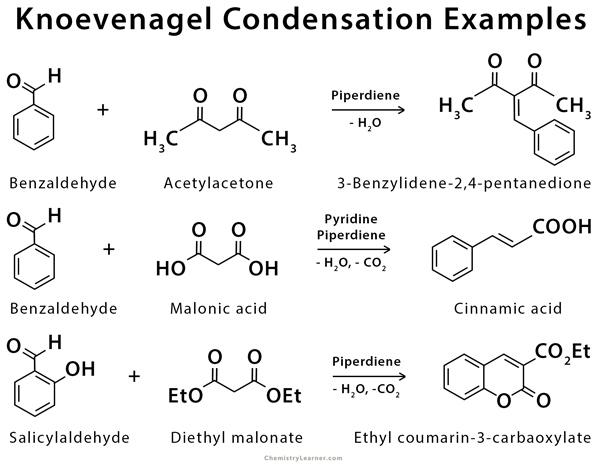
Knoevenagel condensation is used in the synthesis of cinnamic acid and coumarin [4-8].
Mechanism of Knoevenagel Condensation
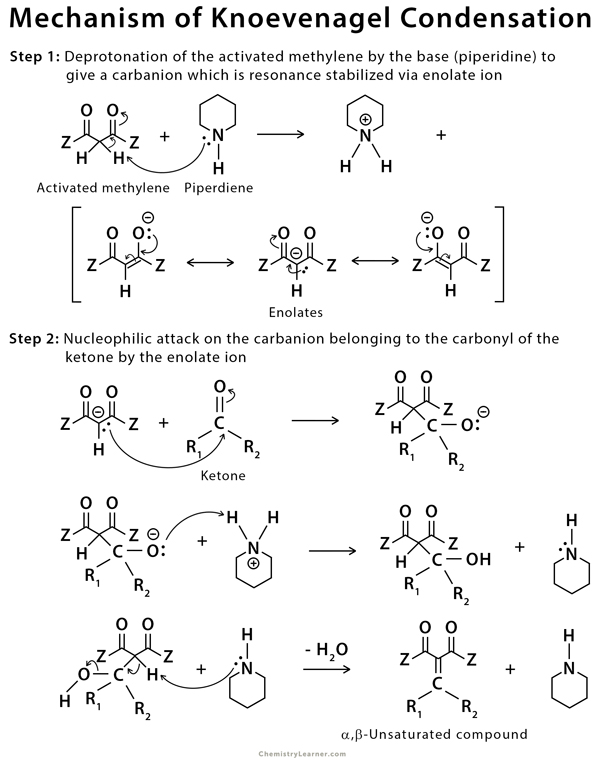
In the first step of this reaction, an amine base deprotonates the complex methylene (usually a diketone) to form a resonance stabilized anion (enolate). This anion then acts as a nucleophile and attaches to the carbonyl acceptor molecule to form an aldol product, which can undergo the dehydration reaction to form the α,β-unsaturated product [2-5,9].
Applications of Knoevenagel Condensation
The product α,β-unsaturated compound is an intermediate in the formation of natural products, therapeutic agents, adequate chemicals, polymers having different functional groups, insecticides and pesticides. In commercial production, the Knoevenagel condensation is a critical step in the production of antimalarial drug lumefantrine, a component of Coartem. Knoevenagel’s use of primary and secondary amines and their salts as catalysts provided a new foundation for the study of amine catalysts [10].
References
- Definition – Chem-station.com
- Definition and mechanism – Name-reaction.com
- Definition and mechanism – Alfa.com
- Definition, examples, and mechanism – Epgp.inflibnet.ac.in
- Definition, examples, and mechanism – Slideshare.net
- Example – Pubs.acs.org
- Example – Tandfonline.com
- Example – Ch.ic.ac.uk
- Mechanism – Synarchive.com
- Applications – Orientjchem.org