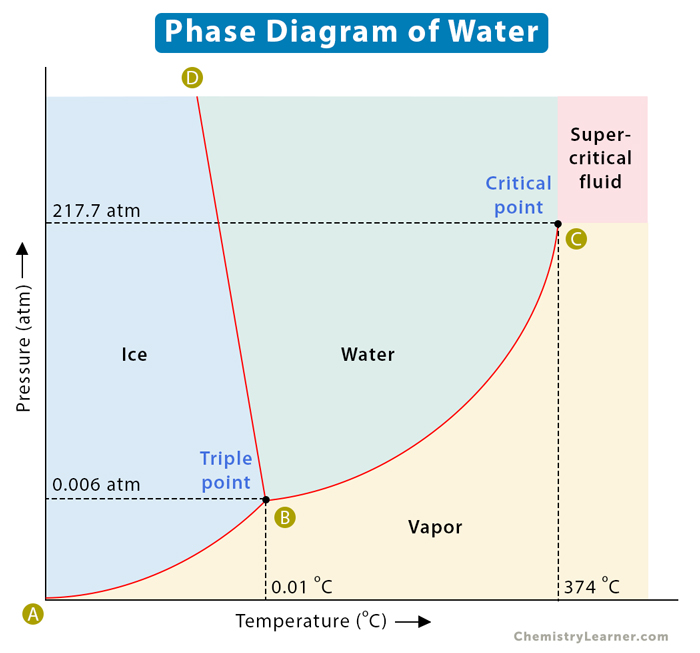
Phase Diagram of Water
A phase diagram is a graphical way of representing a substance’s pressure-volume relationship and identifying its various phases. This relationship is essential because it can predict in which direction phase transformation occurs as we proceed along a constant pressure or temperature line. A water phase diagram indicates what happens to water as it undergoes various phase change processes, as shown below.
Water undergoes phase change under specific conditions of temperature and pressure. These phase changes are as follows:
- Melting: When ice turns into water
- Boiling: When water turns into steam
- Condensation: When steam turns back into water
- Freezing: When water turns back into ice
- Sublimation: When ice is transformed into steam without going through the water phase
The lines on the phase diagram are the lines of equilibrium. Along these lines, any two phases coexist in thermal equilibrium. However, a change in pressure or temperature will cause the phase to change abruptly. The AB line is the ice-vapor line, the BC line is the ice-water line, and the BD line is the water-vapor line. We can use the phase diagram to identify the state where water exists under specific temperature and pressure conditions. For example, water is ice at 50 kPa and – 10 oC.
Water is unique compared to other substances. In other substances, the solid-liquid line is inclined toward the right. However, water’s solid-liquid line is inclined toward the left, meaning that the melting point decreases if pressure increases. It is because solid ice is less dense than liquid water. Hence, ice can float on water. If pressure is applied to ice near its melting point, it transforms into liquid. Water molecules are closer in the liquid than in the solid phase.
Water has a triple point corresponding to the temperature and pressure at which ice, water, and vapor coexist in a stable equilibrium. It also has a critical point – the temperature at which water vapor cannot be forced back into water, no matter how much pressure is applied. The triple point and the critical point are listed in the table below.
| Property | Temperature in Celsius (°C) | Temperature in Kelvin (K) | Pressure in atmosphere (atm) | Pressure in kilo Pascal (kPa) |
|---|---|---|---|---|
| Triple Point | 0.01 | 273.16 | 0.006 | 0.608 |
| Critical Point | 373.99 | 647.14 | 217.7 | 22058.453 |