Zaitsev’s Rule
Definition: What is Zaitsev’s rule?
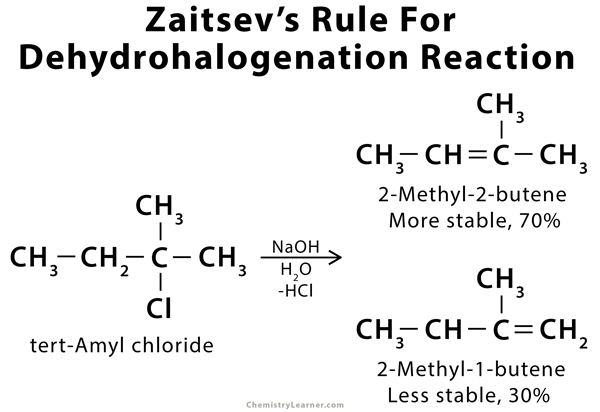
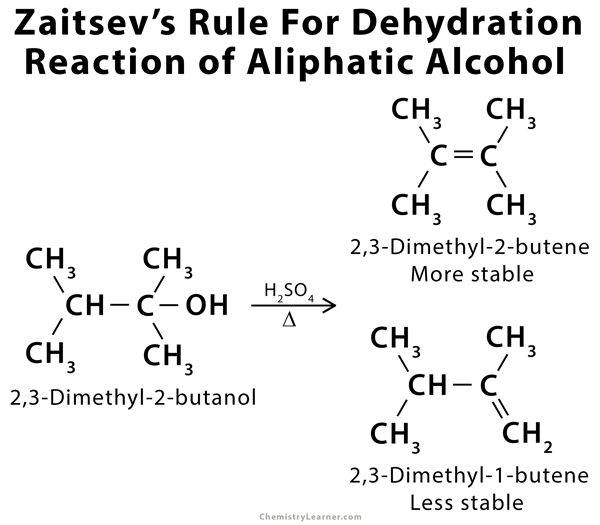
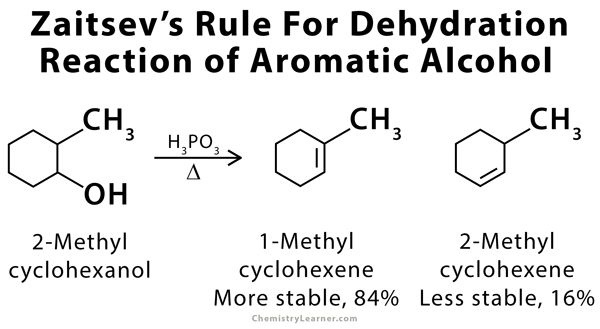
Zaitsev’s rule, also called the Saytzeff Rule, is an empirical rule that can predict the favored alkene product in an elimination reaction. According to Zaitsev, the alkene formed in the highest amount is the one that corresponds to the removal of the hydrogen from that β-carbon that has the fewest hydrogen substituents. In other words, the product with the most substituted carbon in the C=C pi bond will be thermodynamically the most stable. Zaitsev’s rule is mostly applicable to the dehydrohalogenation of alkyl halides and the dehydration of aliphatic and aromatic alcohols. The products formed are known as Zaitsev’s products [1-9].
Elimination Reaction
The elimination reaction is used in the preparation of alkenes. There are three types of elimination reaction – E1 reaction, E2 reaction, and E1cb reaction. Three main steps involved [7,8].
- Removal of proton – deprotonation
- Formation of C=C pi bond
- Breaking of the bond to the leaving group
Anti-Zaitsev’s Rule
In anti-Zaitsev’s rule, the regular Zaitsev’s rule is not followed. Instead, the elimination reaction favors Hofmann rule, and the products obtained from this reaction are known as Hofmann products [9].
FAQs
Q.1. How does Zaitsev’s rule differ from Hofmann rule?
Ans. Unlike Zaitsev’s rule, Hofmann rule states that the most favored and thermodynamically stable alkene during elimination reaction is the one that is least substituted.
Q.2. How does Zaitsev’s rule differ from Markovnikov’s rule?
Ans. Markovnikov’s rule is applicable to addition reaction, whereas Zaitsev’s rule is applicable to elimination reaction.
Q.3. Why are Zaitsev’s products stable?
Ans. Zaitsev’s products are stable because of hyperconjugation with the double bond.
- References
- Definition – Study.com
- Definition – Chem.ucla.edu
- Definition –Chemistry.msu.edu
- Definition – Chem.libretexts.org
- Definition – Chem.libretexts.org
- Definition – Chem.ucalgary.ca
- Elimination reaction – Chem.ucalgary.ca
- Elimination reaction – Home.iitk.ac.in
- Anti-Zaitsev’s rule – Masterorganicchemistry.com





It really hepled me understand the concept alot about the Zaitsev’s rule when saw the explaination and examples.
thanks so much!!