Friedel-Crafts Alkylation
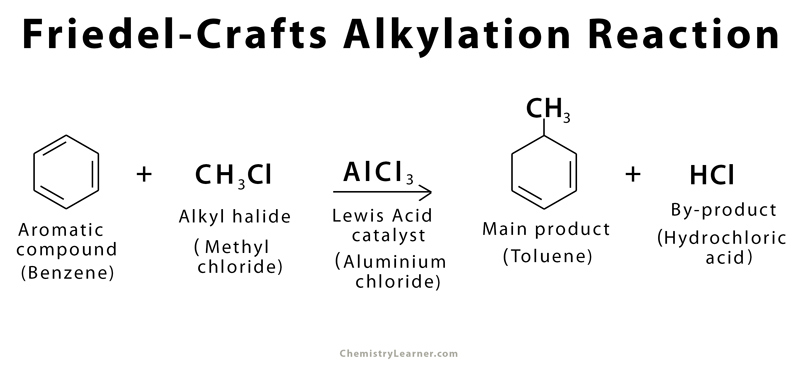
Friedel-Crafts alkylation is defined as a chemical reaction in which an alkyl halide (e.g., alkyl chloride, alkyl bromide) is added to an aromatic ring (e.g., benzene, toluene) in the presence of a Lewis acid catalyst (e.g., aluminum chloride, ferrous chloride). The history of Friedel-Crafts alkylation goes back to 1877 when it was first synthesized by French scientists Charles Friedel and his American co-worker James Crafts using electrophilic aromatic substitution[1].
Example of Friedel-Crafts Alkylation
An example of Friedel-Crafts alkylation is adding a methyl group to a benzene ring to form toluene.
Mechanism of Friedel-Crafts Alkylation
There are a few common reagents used in Friedel-Crafts alkylation. Benzene is (C6H6) typically used as the starting aromatic compound because of the presence of an unsaturated C=C bond, and alkyl chloride is the preferred alkyl halide. Aluminum chloride (AlCl3) is the Lewis acid catalyst.
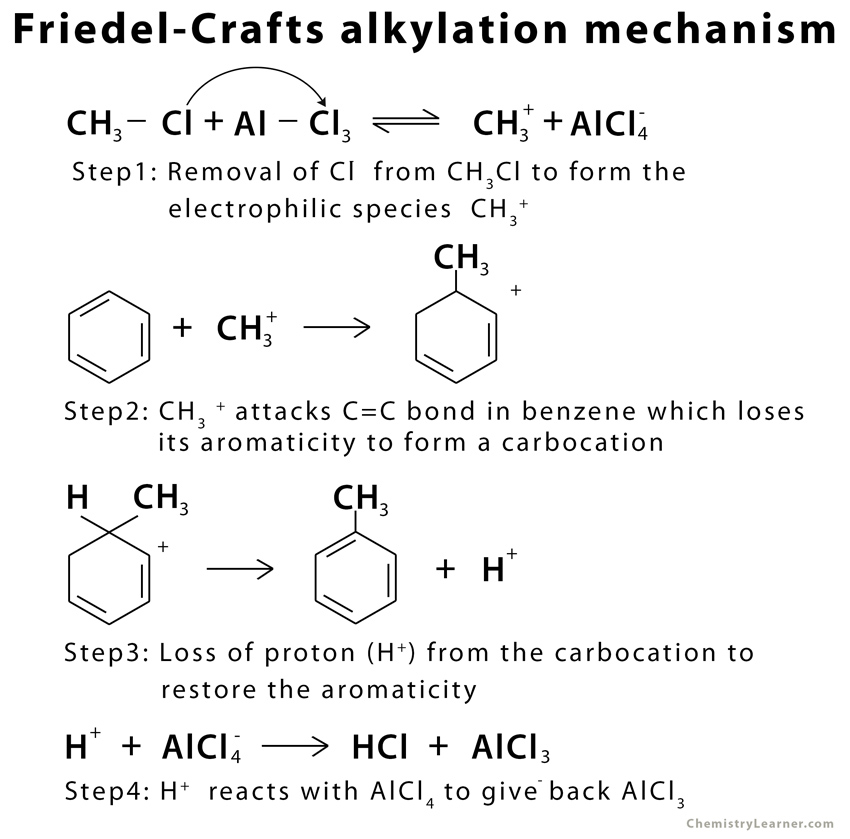
A typical Friedel-Crafts alkylation reaction involves a 4-step mechanism under anhydrous conditions [3].
The above mechanism is an example of intermolecular Friedel-Crafts alkylation. In the case of intramolecular Friedel-Crafts alkylation, the nucleophile (aromatic ring) and the electrophile (alkyl halide) are in the same compound. They are attached to one another through a tether [4].
Biphenyl can also be used as the starting reagent for Friedel-crafts alkylation. In this case, the alkyl halide used is tert-butyl chloride, which reacts with biphenyl to give 4,4’ di-tert-butylphenyl [2].
Limitations and Drawbacks of Friedel-Crafts Alkylation
The basic limitations of Friedel-Crafts alkylation are as follows [5]:
- Carbocation rearrangement – The carbocation rearrangement occurs if the alkyl species added to the ring has more than two carbons.
- Compound limitations – The reaction will not proceed for certain deactivated aromatic compounds like nitrobenzene. Besides, aniline (C6H5NH2) and NHR and NR2 substituted aromatic compounds do not undergo Friedel-Crafts alkylation as they tend to react with the Lewis acid directly.
- Polyalkylation – Adding more than one alkyl group to the benzene ring is called polyalkylation. The presence of the electron-donating alkyl group makes it easier for the benzene ring to undergo further alkylation.
Friedel-Crafts Alkylation vs. Acylation
The Friedel-Crafts acylation was developed to overcome the limitations of Friedel-Crafts alkylation. In this reaction, an acyl group is added to the benzene ring to form an aldehyde or ketone. There are certain differences between Friedel-Crafts alkylation and acylation. Unlike the alkylation reaction, the Lewis catalyst in the acylation reaction gets consumed and must be added in a stoichiometric amount to compensate for the loss. Moreover, Friedel-Crafts acylation has few advantages over alkylation and provides much better control over the formation of stable products [6].
Applications of Friedel-Crafts Alkylation
Friedel-Crafts alkylation reaction forms an important part of the six main electrophilic aromatic substitution reactions, others being chlorination, bromination, nitration, sulfonylation, and acylation. One of the major applications of Friedel-Crafts alkylation is in the total synthesis of natural products and complex molecules, which in turn find their use in various biological activities [7].
References
- History of Friedel-Crafts alkylation – libretexts.org
- Friedel-Crafts alkylation of biphenyl – berkeley.edu
- Mechanism Friedel-Crafts alkylation – com, Chem.ucalgary.ca, Cliffsnotes.com
- Intramolecular Friedel-Crafts alkylation – com
- Limitations of Friedel-Crafts alkylation – libretexts.org
- Friedel-Crafts alkylation vs acylation – com, Masterorganicchemistry.com
- Applications of Friedel-Crafts alkylation – Pubs.rsc.org