Hydrogen Bond
- What is a Hydrogen Bond?
- How is Hydrogen Bond Formed?
- Hydrogen Bond Donor and Acceptor
- Properties and Characteristics of Hydrogen Bond
- Types of Hydrogen Bond
- Importance and Applications of Hydrogen Bonding in Daily Life
- Hydrogen, Ionic, and Covalent Bonds
- Hydrogen Bond vs. Ionic Bond vs. Covalent Bond
- Similarities between Hydrogen and Ionic Bonds
- FAQs
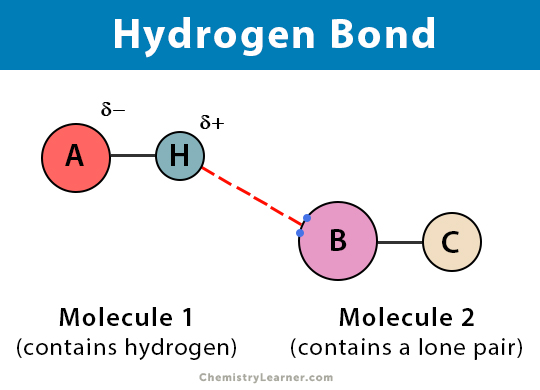
What is a Hydrogen Bond?
A hydrogen bond is a type of chemical bond that involves the electrostatic attraction between a hydrogen atom and an atom containing a lone pair of electrons in a substance. In order for a hydrogen bond to occur, the hydrogen must be bonded to an electronegative atom.
The hydrogen bond is not covalent, although the molecules present in the substance are covalent [1-5].
How is Hydrogen Bond Formed?
When a hydrogen atom is covalently bonded to an electronegative atom, the shared pair of electrons is attracted to the latter. As a result, the hydrogen is slightly positively charged, and the electronegative atom is slightly negatively charged. This property is known as polarity, and such molecules are called polar molecules. When two or more such molecules are present in a substance, the positive end of one molecule is attracted to another molecule’s opposing end. This type of bonding is a hydrogen bond.
Hydrogen Bond Donor and Acceptor
In a hydrogen bond, the donor is usually a strongly electronegative atom such as nitrogen (N), oxygen (O), or fluorine (F) that is covalently bonded to a hydrogen atom. On the other hand, the hydrogen acceptor is an electronegative atom of an adjacent molecule, containing a lone pair involved in the hydrogen bond (example, O, N, Cl, and F).
Properties and Characteristics of Hydrogen Bond
- A weak force of attraction between molecules
- Alters the physical properties of the molecules. For example, it increases the melting and boiling point of a substance, thereby making it less volatile
- Stronger than the weak Van der Waals bond, but weaker than ionic and covalent bonds
- Responsible for solubility of many substances in water
- Responsible for high viscosity and surface tension of the substances
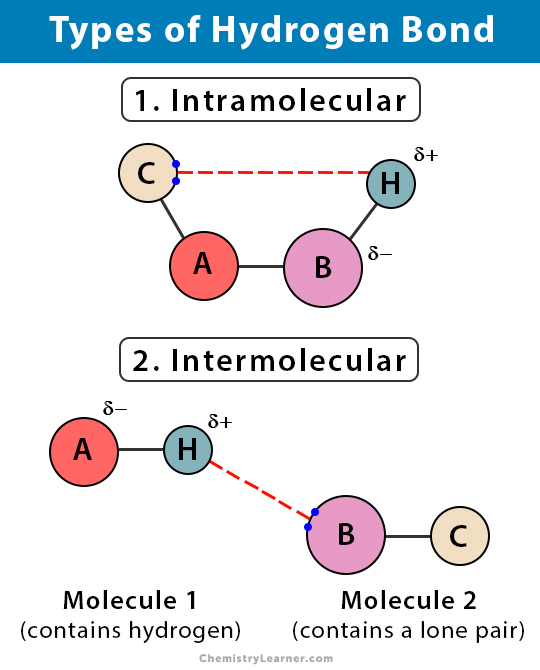
Types of Hydrogen Bond
There are two types of hydrogen bond [1-5].
1. Intramolecular Hydrogen Bond
An intramolecular hydrogen bond occurs within a single molecule. It occurs when two functional groups of a molecule can form hydrogen bonds with each other. For this to happen, both the hydrogen donor and hydrogen acceptor must be present within one molecule. They must be within proximity of each other.
Example
- Ethylene glycol (C2H4(OH)2) has two hydroxyl (OH) groups. The hydrogen bond takes place between the two groups due to molecular geometry.
2. Intermolecular Hydrogen Bond
An intermolecular hydrogen bond occurs between two or more separate molecules in a substance. The hydrogen donors and acceptors are present in positions where they can interact. The intermolecular hydrogen bond’s strength is most often estimated by measuring the equilibria between molecules containing donor and acceptor units in solutions.
Examples
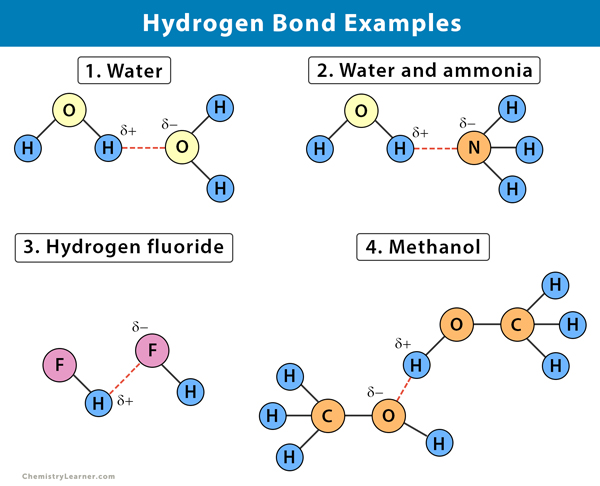
1. Water (H2O)
The water molecule consists of two hydrogen (H) atoms covalently bound to an oxygen (O) atom. Since it is more electronegative than hydrogen, oxygen pulls the shared electrons more closely to itself. This sharing gives the oxygen atom a slightly more negative charge, and the two hydrogens a slightly positive charge. This imbalance causes the water molecule to have a positive and negative side, and hence, water is a polar molecule. The molecules align such that the hydrogen on one molecule will face the oxygen on another molecule. This alignment gives water a greater viscosity and also allows it to dissolve other polar molecules.
2. Ethanol (C2H5OH)
Ethanol is an alcohol that contains an -OH group. It has a hydrogen (H) atom attached directly to an oxygen (O) atom, which still has two lone pairs of electrons like a water molecule. As a result, the OH bond is polar. The hydrogen bond is observed between ethanol molecules, although it is not as effective as water. The hydrogen bonding is limited because there is only one hydrogen in each ethanol molecule with a sufficient positive charge.
3. Ammonia (NH3)
Ammonia consists of three hydrogen (H) atoms connect to a nitrogen (N) atom. Nitrogen, being more electronegative than hydrogen, will attract the shared pair of electrons, making it slightly negative. The hydrogen will be slightly positive, resulting in a hydrogen bond. The amount of hydrogen bonding is limited because each nitrogen only has one lone pair in its outermost shell. There are not enough lone pairs of electrons in a cluster of ammonia molecules to satisfy all the hydrogens. That means that each ammonia molecule can form one hydrogen bond using its lone pair and one involving one of its positive hydrogens. The other hydrogens do not participate in hydrogen bonding. The result is that ammonia has a lower boiling point than water.
4. Hydrogen Fluoride (HF)
Fluorine (F) is a very electronegative element. So, the shared electrons will be attracted strongly towards the fluorine and away from the hydrogen. That leaves the hydrogen with quite a lot of positive charge, and the fluorine quite negative charge. The fluorine also has lone pairs of electrons, which is very strongly attracted to the positive hydrogen. Thus, the reasonably positive hydrogen on one HF molecule will be attracted to one of these lone pairs on a nearby HF molecule.
Importance and Applications of Hydrogen Bonding in Daily Life
Hydrogen bonding is essential in many chemical and biological processes. It can account for many natural phenomena [1,6].
1. Plants
Water can stick to itself and other molecules. The former is known as cohesion and the latter, adhesion. When water droplets fall on a leaf, the hydrogen bonds formed between water molecules are more substantial than the intermolecular forces of adhesion between the water molecules and leaf. This specific property of water explains its high surface tension.
2. Proteins
Intramolecular hydrogen bonding is responsible for the secondary, tertiary, and quaternary proteins and nucleic acids’ structures. The hydrogen bonds assist the proteins, and the nucleic acids form and maintain specific shapes.
3. DNA
The double-helix model of DNA (deoxyribonucleic acid) consists of two intertwined strands held together by a base pair. The hydrogen bonds between the bases on adjacent strands are responsible for this. Because of different bases’ structures, adenine (A) always forms hydrogen bonds with thymine (T). In contrast, guanine (G) always forms hydrogen bonds with cytosine (C) [7].
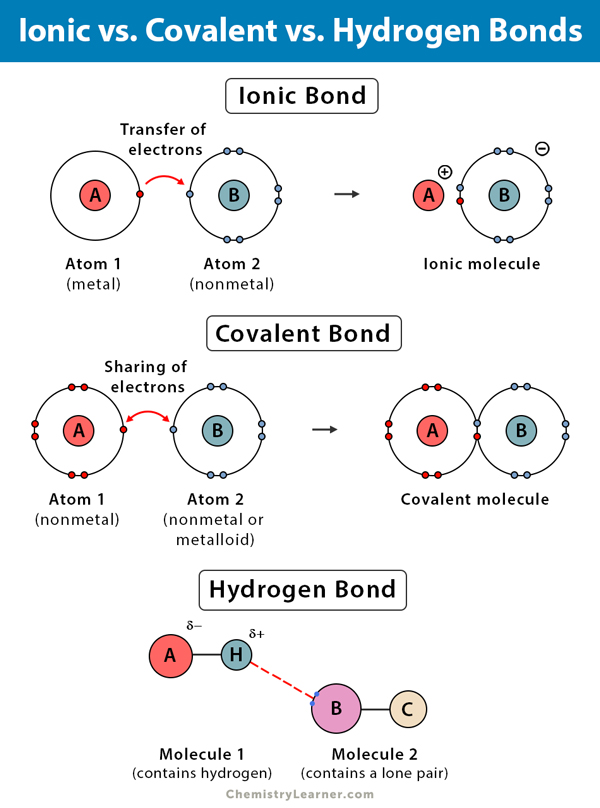
Hydrogen, Ionic, and Covalent Bonds
Hydrogen Bond vs. Ionic Bond vs. Covalent Bond | |||
| Hydrogen Bond | Ionic Bond | Covalent Bond | |
|---|---|---|---|
| Types of atoms | Between a hydrogen atom and an electronegative atom | Between metal and a nonmetal | Between two nonmetals or a nonmetal and a metalloid |
|
Formation |
Electrostatic attraction between hydrogen and an electronegative atom |
Electrostatic attraction between oppositely charged ions |
Sharing electrons between atoms |
|
Type of bond in covalent compounds |
Secondary |
– |
Primary |
|
Strength |
Weak |
Strong |
Strong |
|
Factors affecting the strength |
Strength is high if the electronegativity difference between hydrogen and the atom to which it is covalently bonded is high |
Strength is high with the two atoms have a high electronegativity difference |
Strength is high when the bonded atoms have similar electronegativity |
|
Occurrence |
Polar molecules |
Polar molecules |
Both polar and nonpolar molecules |
|
Conductivity |
Low |
Low, but increases in molten state and solutions |
Low |
|
Does not apply |
Yes |
Yes | |
|
Intramolecular or intermolecular |
Both intramolecular and intermolecular, although the latter is common |
Intramolecular |
Intramolecular |
|
Examples |
The attraction between two water (H2O) molecules and two strands of DNA |
Sodium chloride (NaCl) and magnesium oxide (MgO) |
Ammonia (NH3) and hydrochloric acid (HCl) |
Similarities between Hydrogen and Ionic Bonds
- Involve electrostatic forces
- The electrostatic attraction is between atoms with opposite charges
- Involve polar molecules
- Disrupted by polar solvents, like water
- Ionic bond takes place within a molecule. Hydrogen bond can take place within a molecule.
FAQs
Ans. Acetone (CH3OCH3) does not have hydrogen bonding because no hydrogens bonded directly to the oxygen.
Ans. Soap reduces the surface tension of water by weakening the hydrogen bonds.