Intramolecular Forces
What are Intramolecular Forces
Intramolecular forces, also known as intramolecular interactions, are the forces that arise within a molecule. It is the force responsible for holding the atoms together in a molecule. The conductivity and solubility of substances in the presence of solvents and the physical properties of metals depend on the intramolecular forces. The difference between intramolecular forces and intermolecular forces is that the former occurs between the atoms in a molecule, and the latter occurs between two molecules.
A chemical bond is an intramolecular force. It keeps the molecule stable by holding the atoms together. For example, water (H2O) has two hydrogen atoms bonded to one oxygen atom by two single covalent bonds. As a result, attractive forces occur between each hydrogen atom with the oxygen.
Types of Intramolecular Forces
Intramolecular forces include the following:
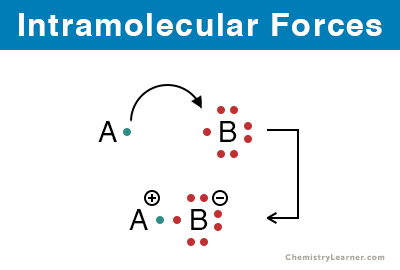
1. Ionic Bond: It is due to the attraction between ions. Ions are formed when an atom loses or gains electrons. The ionic bond is formed between a metal and a nonmetal, where the metal loses electrons, and the nonmetal gains them.
Examples: Sodium chloride (NaCl), potassium iodide (KI), and magnesium oxide (MgO)
2. Covalent Bond: It is due to sharing of electrons between two atoms. Atoms share their outermost or valence electrons to fulfill the octet rule and form bonds. Such a type of bonding occurs between two nonmetals. The covalent bond is the strongest of all intramolecular forces and the most common chemical bond in living organisms.
Examples: Water (H2O), carbon dioxide (CO2), and ammonia (NH3)
3. Metallic Bond: A force that holds atoms together in a metal. The outermost electron shells (atomic orbitals) of each metal atom overlap with many neighboring atoms. As a result, the valence electrons move freely from one atom to another, which results in an attraction between the electron cloud and the positively charged nuclei.
Examples: Sodium (Na), potassium (K), and gold (Au)
References
- Chemical Bonds and Forces – Sites.duke.edu
- Intramolecular & Intermolecular Forces – Learningcenter.unt.edu
- Intermolecular Forces – Chemed.chem.purdue.edu
- Intermolecular vs. Intramolecular Forces – Jove.com
- Intramolecular Forces Chemistry Tutorial – Ausetute.com.au