Endergonic Reaction
What is an Endergonic Reaction
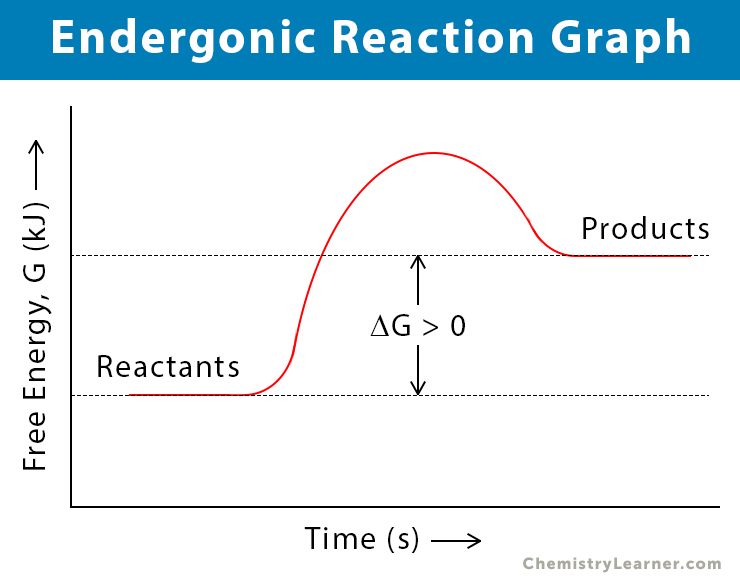
An endergonic reaction is a chemical reaction in which the reaction (system) absorbs energy from its surroundings. It is opposite to the exergonic reaction. The change in Gibbs’ free energy (ΔG) is positive, i.e., the reactants have less free energy than the products. Endergonic reactions are not spontaneous since they require external energy to proceed. These reactions are synthesis (buildup and anabolic). All endothermic reactions are endergonic, but not all endergonic reactions are endothermic.
Endergonic reactions form new chemical bonds that store energy until they are broken to release the energy. However, the chemical bonds that are formed are weaker than the ones that were broken. The endergonic reaction aims to create building blocks of life like DNA and RNA [1-6].
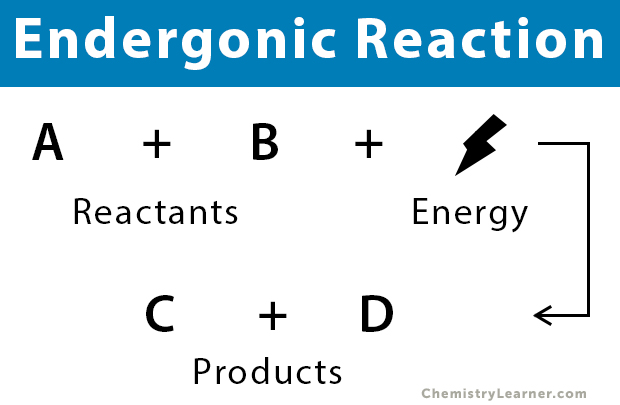
General Equation
Reactants + Energy → Products
Examples of Endergonic Reaction
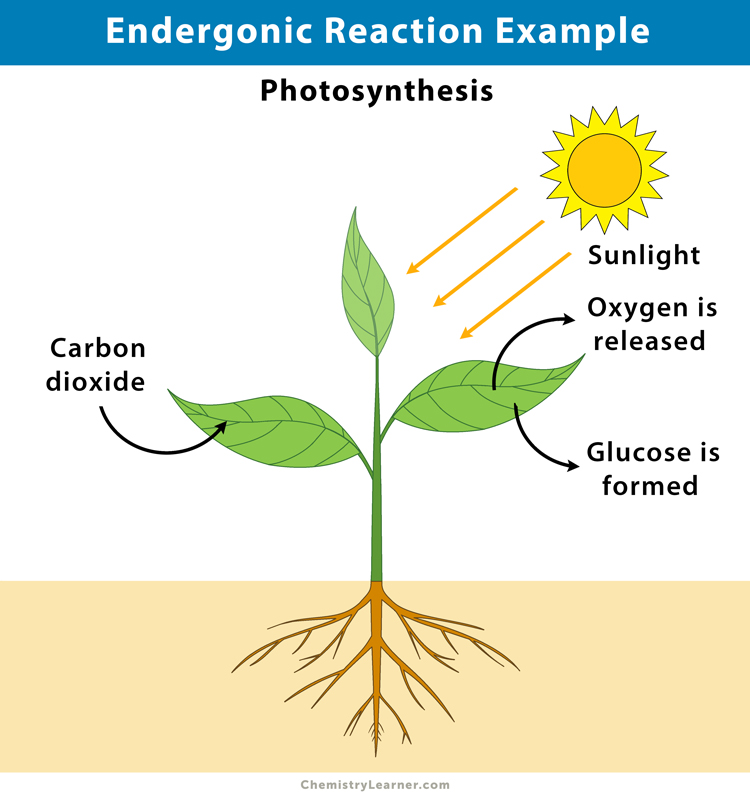
1. Photosynthesis
Photosynthesis is considered an endergonic reaction because plants take in energy from the sun to convert carbon dioxide (CO2) and water (H2O) into sugar (C6H12O6) and oxygen (O2) [7].
6 CO2 (g) + 6 H2O (l) + energy → C6H12O6 (s) + 6 O2 (g)
2. Melting
Water melts into ice by absorbing heat energy from the surroundings.
H2O (ice) + energy → H2O (water)
Other examples include DNA/RNA synthesis, protein synthesis, and fatty acid synthesis. Also, a polymerization reaction is endergonic because it reduces entropy.
3. Electrolysis of water: Energy in the form of an electric current is supplied to water (H2O) to form hydrogen (H2) and oxygen (O2). This process is known as electrolysis and is a redox reaction (oxidation-reduction reaction).
2 H2O (l) → 2 H2 (g) + O2 (g)
4. Formation of nitrogen monoxide: Nitrogen (N2) and oxygen (O2) in the atmosphere combine at normal to high temperatures to form nitrogen monoxide or nitric oxide (NO).
N2 (g) + O2 (g) → 2 NO (g)
5. Ozone formation: Conversion of oxygen (O2) to ozone (O3) is non-spontaneous and endothermic at all temperatures. It is driven by ultraviolet light from the sun.
3 O2 (g) + hν → 2 O3 (g)