Endothermic Reaction
What is Endothermic Reaction?
A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. The heat is absorbed from the surroundings allowing the reactants to transform into products. For example, plants take in energy from the sun and convert carbon dioxide and water into glucose and oxygen. An endothermic reaction causes the surroundings to cool down. That explains why we feel cold when inside a canopy of trees. The endothermic reaction is the opposite of an exothermic reaction [1-6].
General Equation
Reactants + Energy (heat) → Products
Endothermic Process
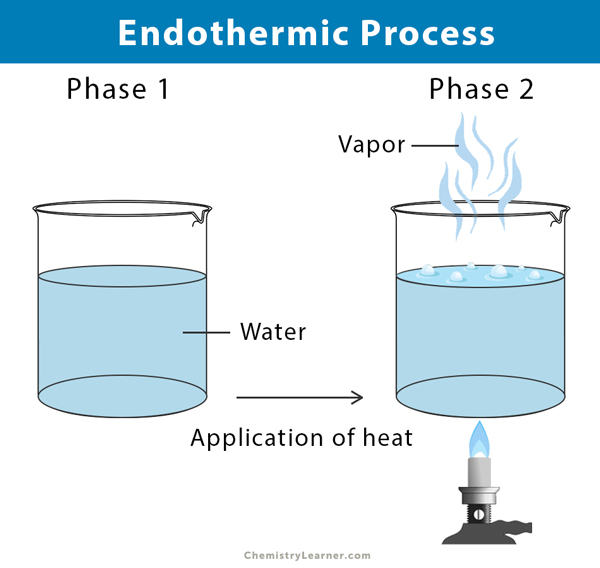
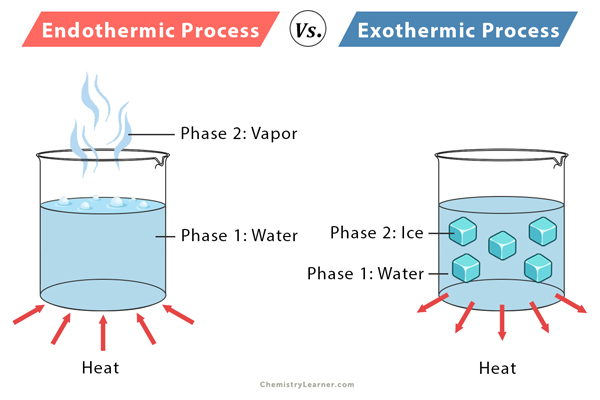
An endothermic process does not involve a chemical reaction. Here, the system takes in heat from the surrounding, transforming a substance from one phase to another. For example, when water boils, it takes heat from a burner, hotplate, or stove and transforms into vapor.
Endothermic Reaction Energy Diagram
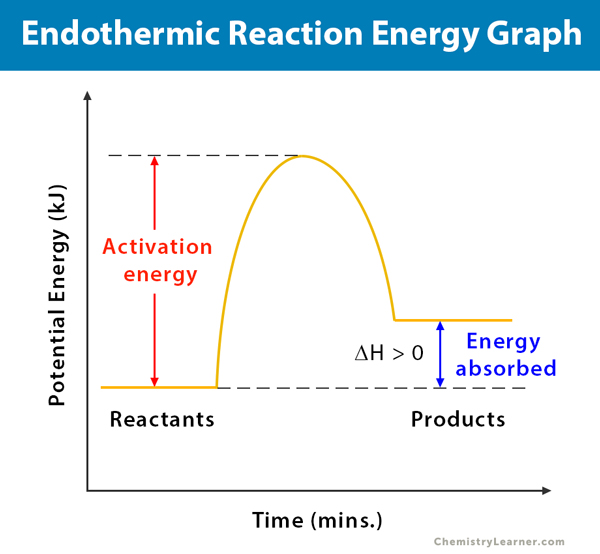
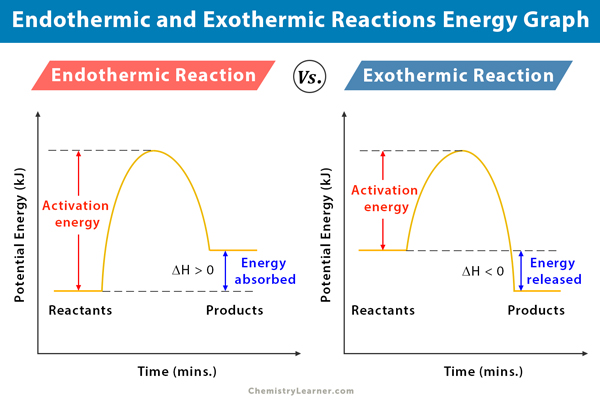
It is possible to represent the endothermic reaction in the form of a graph called the energy diagram. The potential energy of the reaction is plotted along the vertical axis, and time is plotted along the horizontal axis. This graph shows the change in energy as the reaction progresses from reactants to products.
The enthalpy (H) of a reaction is a manifestation of energy. It represents the heat content of a reaction. When a reaction absorbs or emits heat at constant pressure, the enthalpy changes. The change in enthalpy (ΔH) of a reaction can be calculated and defined as the difference between the products’ enthalpy and the reactants’ enthalpy. From the energy diagram of an endothermic reaction, one can see that the products’ energy is higher than that of the reactants. Therefore, ΔH is positive (ΔH>0). From this positive change in enthalpy, one can tell if the reaction is endothermic or not.
During the reaction, few intermediates form. The activation energy of a reaction is the difference between the intermediates’ energy and reactants’ energy. In other words, the activation energy is the energy required for the reaction to overcome a “barrier” and proceed until the transformation is completed. Sometimes, a catalyst is used in a reaction to reduce the activation energy so that the reaction proceeds rapidly [1-6].
What Happens in an Endothermic Reaction?
In an endothermic reaction, the reaction takes place within a system. The system takes heat from the surroundings, making the surroundings colder and the system hotter. The reactants transform into products. They absorb heat energy to overcome the activation energy barrier that allows the reaction to form products.
Most endothermic reactions are not spontaneous, i.e., the reaction will continue as long as heat is supplied. A spontaneous endothermic reaction occurs when the enthalpy changes yield a negative Gibbs’ free energy. Another physical entity, called entropy, also plays a role in the spontaneous reaction. The spontaneity happens when the entropy of the reaction increases by more than the change in enthalpy.
Examples and Applications of Endothermic Reaction and Process
Laboratory Uses
1. Endothermic Chemical Reaction
Here are some examples of endothermic reactions that commonly occur in laboratories. The balanced chemical equations are shown along with the examples [1-6].
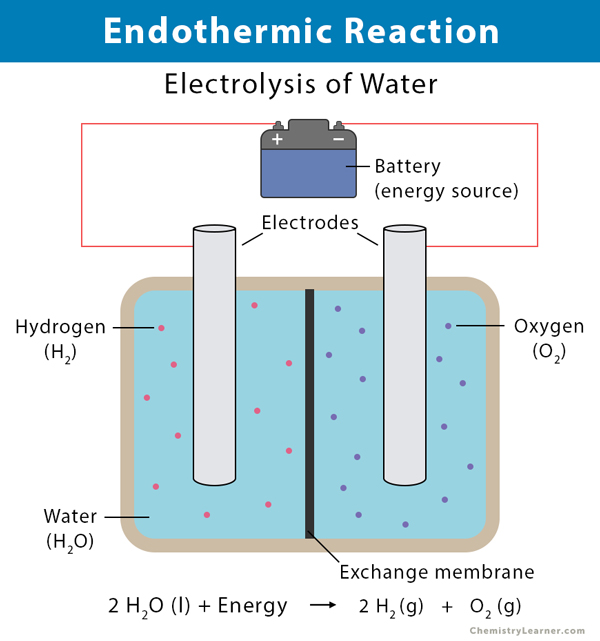
- Decomposition of water: Electrolysis is the process of decomposing water (H2O) into oxygen (O2) and hydrogen (H2) when an electric current is passed through it.
2 H2O (l) + energy → 2H2 (g) + O2 (g)
- Formation of nitric oxide: Nitric oxide (NO) is formed when nitrogen (N2) and oxygen (O2) reacts at high temperature, especially in the upper atmosphere.
N2 (g) + O2 (g) + heat → 2NO (g)
- Decomposition of limestone: Limestone or calcium carbonate (CaCO3) decomposes when heated to a high temperature. The products are quick lime or calcium oxide (CaO) and carbon dioxide (CO2).
CaCO3 (s) + heat → CaO (s) + CO2 (g)
- Disassociation of silver chloride: Silver chloride (AgCl) takes in energy from sunlight and breaks down into silver (Ag) and chlorine (Cl) gas.
2 AgCl (s) + energy → 2 Ag (s) + Cl2 (g)
2. Endothermic Process
An endothermic process undergoes a physical transformation without a chemical reaction. The substance transforms from one form to another by absorbing heat or disassociating in solutions.
- Sublimation of solid carbon dioxide or dry ice
CO2 (s) + heat → CO2 (g)
Everyday Life Examples
There are some common endothermic reactions and processes that are found in real life. We can feel the importance of these reactions in our daily lives. Besides, some everyday household items undergo endothermic reactions.
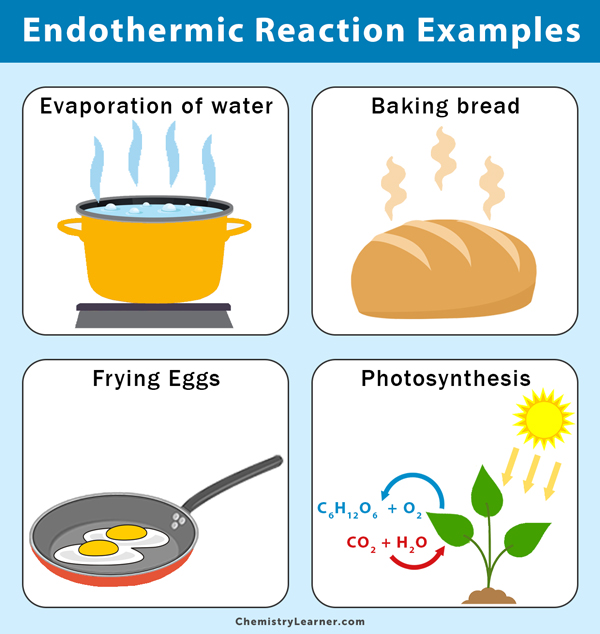
- Photosynthesis: A photochemical reaction in which plants take in energy from the sun to convert carbon dioxide (CO2) and water (H2O) into sugar (C6H12O6) and oxygen (O2).
6 CO2 (g) + 6 H2O (l) + heat → C6H12O6 (s) + 6 O2 (g)
- Baking of bread: Baking requires mixing various ingredients and heating the mixture in an oven over a specific time. It involves the chemical transformation of the soft dough and butter using the heat from the oven.
- Cold pack: A cold pack consists of water (H2O) and ammonium nitrate (NH4NO3) separated by a thin film and placed in a plastic bag. When the pack is squeezed, the film breaks, and ammonium nitrate dissolves in water as ammonium (NH4+) cations and nitrate (NO3–) anions to give the instant cold feeling.
NH4NO3(s) + H2O (l) + heat → NH4+ (aq.) + NO3– (aq.) + H2O (l)
This reaction is an example of salts dissolving in water. It also demonstrates that endothermic reactions can be very safe.
- The reaction between baking soda and vinegar: When baking soda (NaHCO3) is added to vinegar (CH3COOH), the two react with one another to produce sodium acetate (CH3COONa), carbon dioxide (CO2) gas, and water (H2O). It takes more energy to break the baking soda and vinegar apart than the energy required to form the carbon dioxide, sodium acetate, and water. This reaction is a standard treatment to clean clogged water pipes.
NaHCO3 (s) + CH3COOH (l) + heat → CH3COO– Na+ (aq.) + CO2 (g) + H2O (l)
- Evaporation of water: Water evaporates into vapor when boiled. When a body of water is exposed to the sun for a long time, it dries up. This phenomenon is commonly observed in nature when high temperatures dry up lakes.
H2O (l) + heat → H2O (g)
- Sublimation of naphthalene or mothballs: Naphthalene balls are everyday household items used to keep clothes fresh. When naphthalene balls are stored in a wardrobe for a long time, they sublimate.
C10H8 (s) + heat → C10H8 (g)
This reaction indicates that sublimation is an endothermic process.
Endothermic and Exothermic Reactions
An exothermic reaction is the opposite of an endothermic reaction. In an exothermic reaction, the reactants transform into products and release heat. In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0).
The criteria in terms of temperature and the impact on the environment for exothermic and endothermic reactions are as follows:
- An exothermic reaction releases heat and warms the environment.
- An endothermic reaction absorbs heat and cools the environment.
Investigating endothermic and exothermic reactions aims to estimate energy changes in chemical reactions. Suppose the products have higher total bond energy than the reactants. In that case, the reaction will be exothermic. The opposite will hold good for an endothermic reaction. In general, bond breaking is considered to be endothermic, and bond-forming is exothermic.
Endothermic vs. Exothermic Reactions | ||
|
|
Endothermic Reaction | |
|
Definition |
The system absorbs heat from surroundings |
The system releases heat to surroundings |
|
Temperature of the surroundings |
Decreases as the reaction progress (feels cold) |
Increases as the reaction progress (feels hot) |
|
Enthalpy of reactants |
Lower than that of products |
Higher than that of products |
|
Enthalpy change of the reaction |
Positive (ΔH>0) |
Negative (ΔH<0) |
|
Spontaneous or non-spontaneous |
Mostly non-spontaneous |
Mostly spontaneous |
|
Examples |
Melting of ice, photosynthesis, evaporation, cooking an egg, and splitting a gas molecule |
Explosions, making of ice, rusting of iron, setting of concrete, and nuclear fission and fusion |
FAQs
Ans. The enthalpy of neutralization (ΔHN) is negative, and hence, a neutralization reaction is exothermic.
Ans. In an endothermic reaction, the enthalpy change is positive. In an endergonic reaction, the change of Gibbs’ free energy is positive. The two are different.