Elimination Reaction
Definition: What is Elimination Reaction?
The elimination reaction is an organic reaction in which two substituents are removed from a molecule to form a new product. The process takes place in the presence of acid, base, metal, and sometimes through heating. This process makes it possible to synthesize unsaturated (double or triple bond between carbon atoms) organic compounds from saturated (single C-C bonds) ones. The elimination reaction is mechanically the reverse of the addition reaction [1-3].
The elimination reaction involves three fundamental steps [4].
- Removal of proton
- Formation of C=C pi bond
- Breaking of the bond to the leaving group
Nomenclatures Used in Elimination Reaction
- When a hydrogen atom is removed from the compound as a proton (H+), the process is called deprotonation.
- If a halogen is removed, then it is called dehalogenation.
- If both hydrogen and halogen are removed, then it is called dehydrohalogenation.
- If hydrogen and oxygen are removed together, as in the case of alcohols, then it is a dehydration reaction or β-elimination.
Types of Elimination Reaction
There are three types of elimination reaction [4 – 5]
1. E1 type
- two-step removal mechanism process
- also known as unimolecular elimination
- formation of an intermediate
- the reaction rate is proportional to the concentration of the compound to be transformed – first-order kinetics
- regioselective and follows Zaitsev’s rule, i.e., favors the formation of Zaitsev product
2. E2 type
- one step removal mechanism process
- also known as bimolecular reaction
- carbon-leaving group and carbon-hydrogen bonds break off simultaneously
- the reaction rate is proportional to both the compound to be transformed and the transferring agent – second-order kinetics
- stereoselective and regioselective
3. E1cb type
- two-step elimination mechanism
- also known as unimolecular conjugate base elimination
- the compound has an acidic hydrogen atom and a weak leaving group (e.g., -OH)
- standard in the dehydration reaction
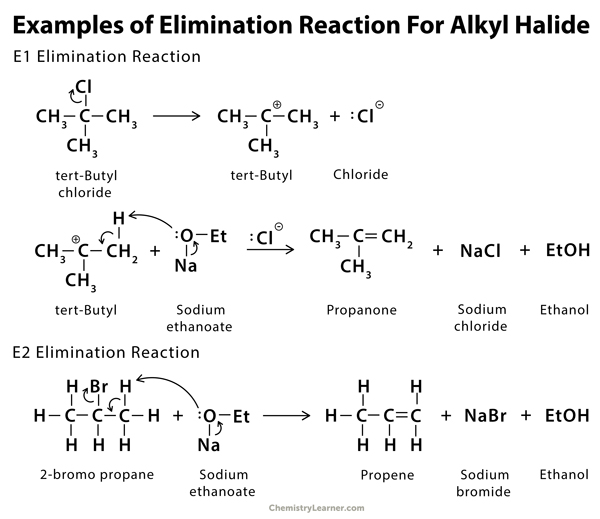
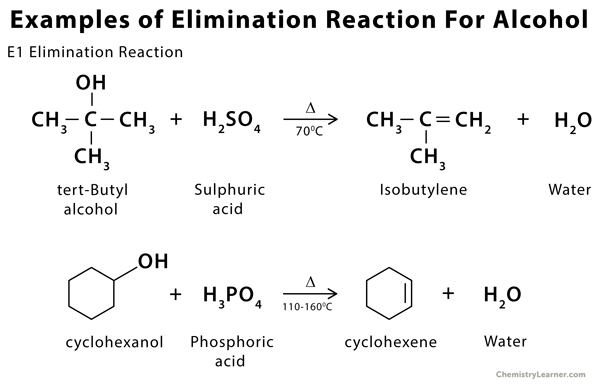
Examples of Elimination Reaction [2, 6]
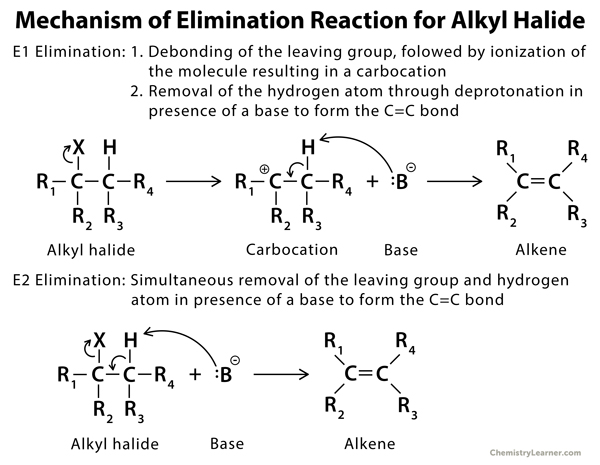
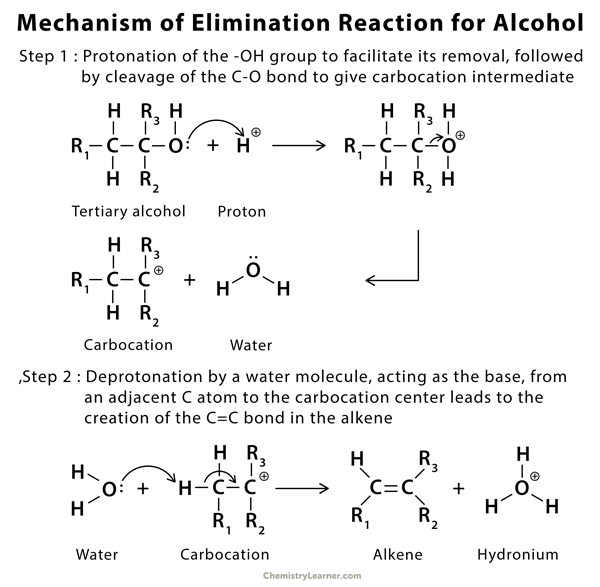
Mechanism of Elimination Reaction [4 – 12]
Conditions of Elimination Reaction
Elimination reactions are usually favored at high temperatures. For the dehydrohalogenation reaction, a strong base is required.
E1 vs. E2 Reaction
Both E1 and E2 reactions are types of elimination reactions. The E2 and E1 mechanisms differ in the timing of bond cleavage and bond formation, which is analogous to the SN1 and SN2 reactions. The strength of the base is the most important factor in determining the mechanism for elimination. Strong bases favor the E2 mechanism, while weak bases favor the E1 mechanism. Here are the differences between E1 and E2 reactions [12].
| E1 Reaction | E2 Reaction | |
| Mechanism | Two-steps | Concerted |
| Order of reaction | First (unimolecular) | Second (bimolecular) |
| Rate of equation | R = kr [substrate] | R = kr [substrate][base] |
| Stereochemistry | Not stereospecific | Stereospecific |
| Base | Usually weak (H2O, ROH, R2NH) | Strong (OH–, RO–, R2N–) |
| Leaving group | Essential and must be good | Not important |
| Solvent | Polar protic | Wide range |
| Examples of substrate | 2-methyl-2-butanol and 2-methyl-2-butene | 2-chloro-2-methyl butane and 1-propanol |
FAQs
Q.1. Where is the elimination reaction applied?
Ans. Application of elimination reaction can be found in Hofmann elimination to synthesize alkene during which the leaving group is a quaternary amine.
Q.2. What is the difference between a substitution and elimination reaction?
Ans. In the case of the elimination reaction, the agent responsible for elimination merely eliminates the group of atoms, whereas, in a substitution reaction, the agent substitutes one replacement group with another.
- References
- Definition – Britannica.com
- Definition – Chem.libretexts.org
- Definition – Siyavula.com
- Definition and mechanism – Byjus.com
- Definition and mechanism – Nptel.ac.in
- Examples – Chem.libretexts.org
- Mechanism – Iitk.ac.in
- Mechanism – Cliffsnotes.com
- E1 reaction mechanism – Chem.ucalgary.ca
- E1 reaction mechanism –Masterorganicchemistry.com
- E2 reaction mechanism – Chem.libretexts.org
- E2 reaction mechanism –Chem.ucalgary.ca