Enantiomers
Enantiomers are molecules that have the same molecular formula and connectivity of atoms but differ in their spatial arrangement. More precisely, enantiomers are mirror-image isomers of each other, just like our left and right hands are mirror images of one another. This concept of mirror symmetry is essential for enantiomers. Thus, enantiomers are a subset of stereoisomers characterized by their mirror-image relationship [1-4].
Characteristics
Enantiomers possess distinct characteristics that set them apart from other isomers. Let us delve into their key characteristics [1-7].
- Chirality: Enantiomers exhibit chirality, meaning they are inherently asymmetric. Chiral molecules lack a plane of symmetry. Thus, they cannot be superimposed onto their mirror image. This inherent lack of being superimposable sets enantiomers apart from other isomers.
- Identical Physical Properties: Enantiomers share identical physical properties such as melting point, boiling point, and solubility. These properties are determined by the overall molecular structure and composition, which remain the same in enantiomers.
- Opposite Optical Activity: One of the most striking features of enantiomers is their interaction with polarized light. Enantiomers rotate plane-polarized light in opposite directions. One enantiomer will rotate it clockwise, a property known as dextrorotation (d or +), while the other will rotate it counterclockwise, called levorotation (l or -). This property is crucial in distinguishing between the two forms.
When two enantiomers exist in equal proportions, they are mutually referred to as a racemic mixture. A racemic mixture does not rotate polarized light because the optical activity of each enantiomer is canceled by the other.
How to Draw Enantiomers
Drawing enantiomers allows chemists to analyze and predict the behavior of chiral molecules. At the core of drawing enantiomers is identifying the chiral center in the molecule. A chiral center is an atom, typically carbon, bonded to four different substituents. This arrangement results in the molecule’s chirality, making it exist as a pair of enantiomers [3].
Enantiomers are often represented using Fischer projections or wedge-dash diagrams to depict their three-dimensional structures visually. These representations help illustrate the spatial arrangement of atoms in the molecule, making it easier to identify their mirror-image relationship.
Once the enantiomer is represented, the next step is to draw the mirror image. To do this, reverse the arrangement of substituents around the chiral center. Suppose the original molecule had a substituent in the upper-right position. In that case, the mirror image will have it in the upper-left position and vice versa.
The step is followed by checking if the two molecules can be superimposed. If they cannot be superimposed and are mirror images of each other, then they are enantiomers. Finally, label the enantiomers as R (representing “right”) and S (representing “left”).
Enantiomers Examples
Examples of enantiomers can be found in various facets of chemistry. We shall discuss three prominent examples [1-4].
Lactic Acid
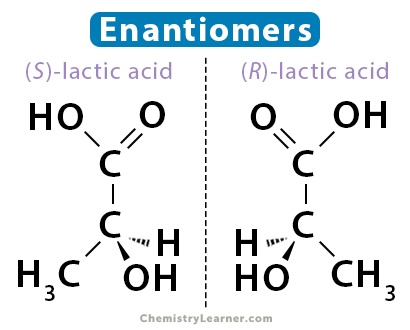
Lactic acid is a well-known compound in living organisms, particularly in biochemistry. It exists in two enantiomeric forms: S-lactic acid and R-lactic acid. These enantiomers have the same chemical formula, C3H6O3, but their molecular structures differ in the arrangement of atoms. Lactic acid is a chiral molecule because it has a carbon atom bonded to four different groups: a hydrogen atom (H), a carboxyl group (–COOH), a methyl group (–CH3), and a hydroxyl group (–OH). Lactic acid enantiomers are crucial in biological processes and affect the human body.
Alanine
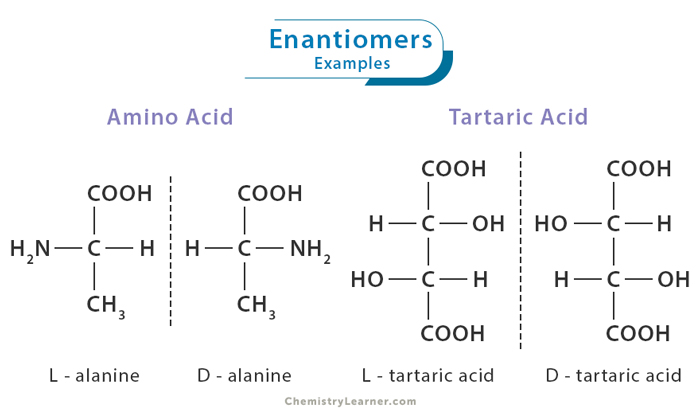
Alanine exists in two enantiomeric forms: L-alanine and D-alanine. These enantiomers have the same chemical formula and connectivity of atoms but differ in the arrangement of their atoms in three-dimensional space. In the case of alanine, the chiral center is the carbon atom known as the alpha carbon (α-C). In L-alanine, the amino group (-NH2) is oriented to the left, while D-alanine is oriented to the right. This subtle difference in spatial arrangement has profound consequences in biological systems.
Tartaric Acid
Tartaric acid exists in two enantiomeric forms: D-tartaric acid and L-tartaric acid. While they share the same chemical formula and most physical properties, they have opposite optical activities. D-tartaric acid rotates polarized light clockwise (dextrorotatory), while L-tartaric acid rotates it counterclockwise (levorotatory). The significance of tartaric acid’s enantiomers lies in their ability to affect the taste and quality of wine.
Enantiomers vs. Diastereomers
Enantiomers and diastereomers are two types of stereoisomers with distinct characteristics and properties that set them apart [5-7].
While enantiomers are non-superimposable mirror images of each other, diastereomers do not share a mirror-image relationship. They are any two or more stereoisomers that are not enantiomers. They differ in spatial arrangement at one or more chiral centers but maintain the same configuration at others. Here are some of their characteristics:
- They often have different physical properties, including melting points, boiling points, and solubilities. It can facilitate their separation.
- They can exhibit different chemical reactivity due to their distinct spatial arrangements, allowing for selective reactions in organic synthesis.
- They may or may not exhibit different optical activities.
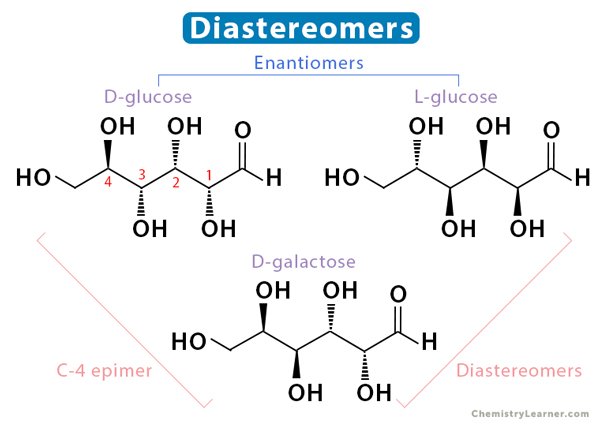
An example of diastereomers is L-glucose and D-galactose.