Physical Properties
A physical property is a feature of a substance that can be measured without altering the identity of that substance. During the measurement, the substance does not change its chemical composition nor convert into an entirely new substance. For example, silver can be molded into thin sheets because it has a property known as malleability. Salt dissolves in water due to a property called solubility. Neither silver nor salt changes into any other substance [1-4].
Examples of Physical Properties
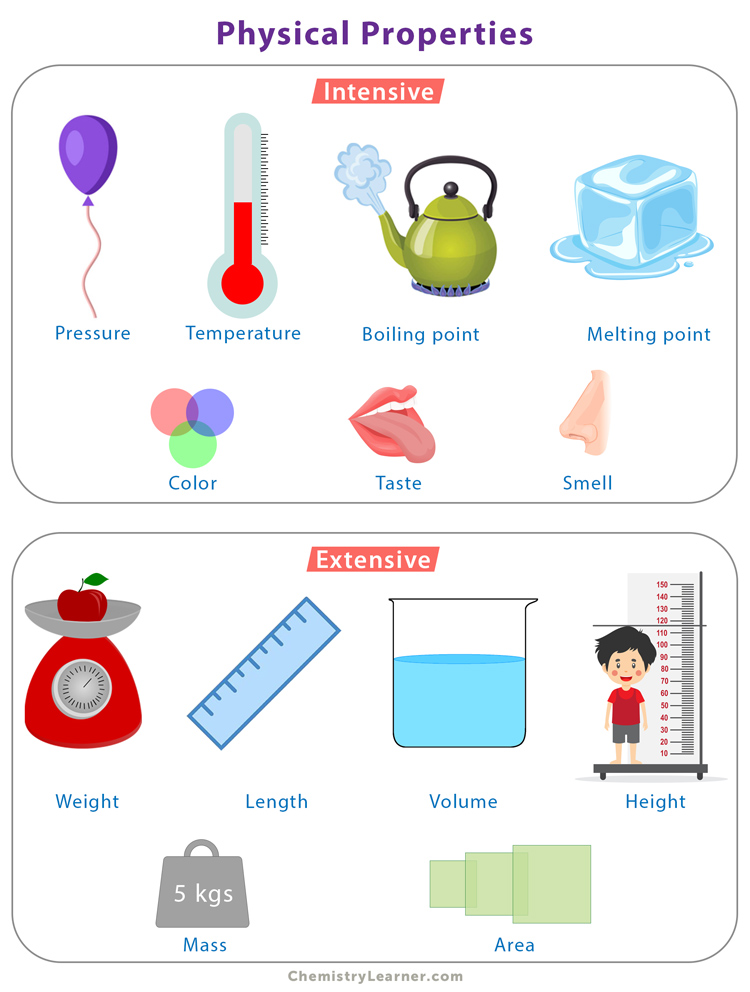
There are two types of physical properties – intensive and extensive [1-6].
Intensive Properties
Intensive properties are independent of the substance’s volume or size. They do not depend on the amount of matter present and are called bulk properties. Some examples of intensive properties are:
1. Pressure – The force applied per unit area.
2. Temperature – Measures the relative hotness or coldness.
3. Concentration – The amount of substance in a mixture.
4. Melting point – The temperature at which a solid converts into a liquid.
5. Boiling point – The temperature at which a liquid converts into a gas.
6. Density – The weight per unit volume.
7. Color – A result of light-induced electron excitation in the molecules.
8. Odor – The feature that stimulates the sense of a characteristic smell.
9. Taste – The sensation of flavor perceived in the mouth.
10. Ductility – The ability to draw into a thin wire.
11. Malleability – The ability to draw into thin sheets.
12. Solubility – The ability to form a solution.
13. Conductivity – The ability to conduct electricity.
Extensive Properties
Extensive properties depend on the amount of matter present in the substance. The size of the substance determines its extensive properties. The value of an extensive property of a substance is equal to the sum of the extensive properties of different parts of the substance. Below are some examples of extensive properties.
1. Mass – The amount of matter present.
2. Weight – The mass multiplied by the acceleration due to gravity.
3. Length – Measures how long a substance is.
4. Breadth – Measures how wide a substance is.
5. Area – The product of the length and breadth.
6. Height – Measures how tall a substance is.
7. Volume – The product of the length, breadth, and height.
FAQs
Ans. The number of protons determines the physical properties of an atom.
Ans. No, reactivity is not a physical property. It is a nuclear property.
Ans. No, flammability is not a physical property. It is a chemical property.
Ans. Yes, viscosity is a physical property. It measures how fast or slow a liquid flows.
Ans. Limestone is a soft sedimentary rock, often grey or white.




IT’S NOT A PHYSICAL SUBSTANCE
We do not understand the comment.