Fenton Reaction
Definition: What is the Fenton Reaction?
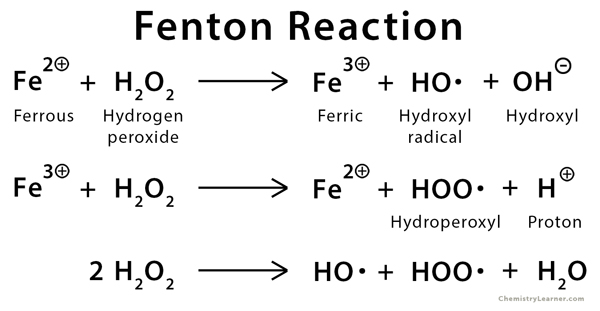
The oxidation of organic substrates by iron (II) and hydrogen peroxide (typically iron (II) sulfate, FeSO4) is called the Fenton reaction. The reagent used for this purpose is known as Fenton’s reagent, which is accepted as one of the most effective methods for the oxidation of organic pollutants. Fenton’s reagent is effective in treating various industrial wastewater components that include aromatic amines, a wide variety of dyes, pesticides, surfactants, and explosives. It is also used to treat many other water-polluting substances like phenols, formaldehyde, BTEX, and rubber chemicals [1,2].
The history of Fenton reaction goes back to 1894 when British chemist H.J.H. Fenton first developed it. He first observed the oxidation of tartaric acid by hydrogen peroxide in the presence of ferrous iron ions. Although Fenton’s reagent is known for a long time, its application as an oxidizing agent for destroying hazardous organics was not applied until the late 1960s.
Preparation of Fenton Reagent
During the preparation of Fenton reagent, the addition of iron and hydrogen peroxide generates free radicals, which then engage in further reactions. These radicals, particularly hydroxyl radical, oxidizes organic compounds. This reaction takes place rapidly and exothermically and results in the oxidation of contaminants to primarily carbon dioxide and water [2].
Application of Fenton Reaction
As mentioned before, the Fenton reaction is used in wastewater treatment for the following [3]:
- Organic pollutant destruction
- Toxicity reduction
- Biodegradability improvement
- BOD/COD removal
- Odor and color removal
- Destruction of resin in radioactive contaminated sludge
Requirement of Fenton Reaction
The pH of the reaction should be between 3 and 6. At high pH, iron will precipitate in Fe(OH)3 and decompose hydrogen peroxide to oxygen. Hydrogen peroxide should be added slowly in order to keep the pH within control [3].
References
- Definition – uni.opole.pl
- Definition – com
- Application – Lenntech.com






Good Morning, I am a 5th year biomedical sciences graduate student at the University of North Dakota. I am writing my dissertation and I would like to ask for permission to use your Fenton reaction image in my dissertation. Thank you for your time.
Warm regards,
Koffi
Sure, you can. As long as you cite us.