Galvanization
Galvanization is a process that involves applying a protective zinc coating to steel or iron to prevent corrosion. This technique has been widely used for many years due to its effectiveness in increasing the lifespan and durability of metal structures. Galvanization is typically achieved through a hot-dip galvanizing process, where the metal is immersed in molten zinc, allowing the zinc to bond with the surface of the metal. [1-4]
What is the Purpose of Galvanization
The primary purpose of galvanization is to provide corrosion protection. When steel or iron is exposed to moisture and oxygen, it can undergo a chemical reaction known as oxidation, leading to rust formation. By applying a layer of zinc through galvanization, the zinc acts as a sacrificial anode, corroding instead of the underlying metal. It effectively prevents rust from forming and extends the lifespan of the coated material. [2,4]
Examples and Applications of Galvanization
One prominent example of galvanized products is galvanized steel structures. These structures are widely used in construction, infrastructure development, and manufacturing industries. Galvanizing steel helps protect it from corrosion, extending its lifespan and ensuring structural integrity. Galvanized steel structures are ideal for outdoor applications and can sustain harsh weather conditions. [2,3]
Another commonly used galvanized product is galvanized wire fences. These fences are extensively employed in residential, commercial, and agricultural settings for their durability and strength. Galvanizing the wire provides a protective zinc coating that prevents rusting and corrosion, making the fence long-lasting even in challenging environments.
Galvanized pipes are also widely utilized in plumbing applications. The zinc coating on these pipes is a barrier against corrosive elements in water or soil, reducing the risk of pipe degradation over time. It makes galvanized pipes popular for residential and industrial plumbing systems.
Types of Galvanization
Several galvanization techniques are available, each with unique characteristics and advantages. [2,3]
1. Hot-dip galvanizing is a widely used technique that involves immersing the metal in a bath of molten zinc. The process creates a thick layer of zinc coating on the surface, providing excellent corrosion protection. Due to its durability, this method is commonly used for large structures and outdoor applications.
The hot-dip galvanizing process involves three primary stages: surface preparation, galvanization, and post-treatment. Its inherent simplicity provides a significant edge over alternative methods for corrosion protection.
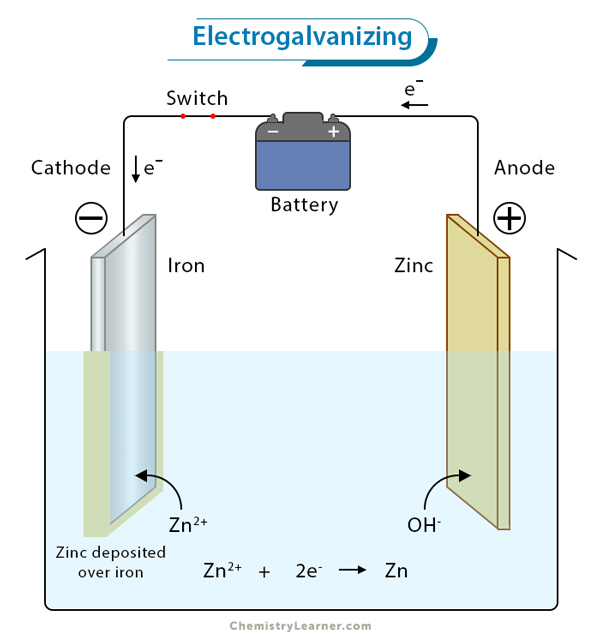
2. Electrogalvanizing, on the other hand, involves applying a thin layer of zinc to the metal surface through an electroplating process. This technique offers precise control over the thickness of the coating and is often utilized for smaller components or products that require a smooth finish.
3. Sherardizing is a unique form of galvanization where the metal is heated in a zinc dust container. The heat causes diffusion between the zinc and the metal surface, resulting in a durable alloy coating. Sherardized coatings are known for their exceptional resistance to corrosion and abrasion.
4. Galvannealing is a process that combines galvanizing and annealing to create a zinc-iron alloy coating on steel. This process helps to enhance steel’s corrosion resistance while improving its paintability and formability. During galvannealing, the steel is coated with a layer of zinc and then heated to a specific temperature, allowing the zinc to alloy with the iron in the steel. It results in a durable and uniform coating that protects against rust and other environmental factors. Galvannealed steel is commonly used in automotive, construction, and appliance industries, where strength and corrosion resistance are required.