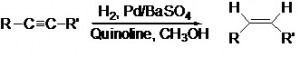Lindlar Catalyst
A catalyst is a substance which alters or accelerates the rate of any chemical reaction without itself undergoing any change. Normally a catalyst is used in smaller amounts compared to the reactants or participants in the reaction.
Lindlar catalyst is a heterogenous catalyst that consists of palladium deposited on calcium carbonate and treated with various forms of lead. A Heterogenous catalyst is a catalyst which is always in a different phase or state (solid, liquid or gas solution) with that of the reactants.
The name “Lindlar” has been given after its inventor Herbert Lindlar. The addition of lead is done in order to deactivate the palladium at certain sites. This is often denoted as “poisoned catalyst” due to the presence of lead. A catalyst becomes poisonous when its effectiveness starts diminishing in the presence of another chemical substance which is called as catalyst poison.
Various catalyst poisons like lead acetate and lead oxide are used to poison the palladium. Usually the palladium content is only 5% of the total weight of the catalyst. The catalyst is used for hydrogenation of alkynes to alkenes.
Lindlar catalyst Formula
Pd/CaCO3
Lindlar catalyst Structure
Lindlar catalyst Properties
Specific surface area- 150-260 m2/g
Impurity < 0.5%
Water content< 5%
pH- 8
Lindlar catalyst Preparation
Normally it’s prepared by reduction of palladium chloride in a mixture of calcium carbonate followed by addition of lead acetate. Finally a catalyst is obtained with a large surface area which increases its reactivity. As the catalyst is used for reduction of alkynes to alkenes, further reduction to alkanes is inhibited by adding quinoline. Thus quinoline acts as a deactivator to increase the selectivity of the catalyst.
Lindlar catalyst Mechanism
Hydrogenation of alkynes to alkenes involves the presence of molecular Hydrogen (H2) which reduces the alkynes to alkenes. The Hydrogen (H2) atoms get added in pairs to the alkenes where the triple bond of the alkynes gets reduced to a double bonded alkene. But as mentioned before, further reduction to a single bond is generally obstructed. Moreover reduction of alkenes to alkanes is faster than the reduction to alkenes due to which quinoline is added.
In the above Hydrogenation reaction the hydrogen atom gets added to the same side (cis) of the alkyne and giving rise to cis alkenes through syn addition (Addition of two substituents in the same side of a double or triple bond resulting in decrease of the number of bonds). Both Hydrogen and alkyne are tightly bound to the large surface of the catalyst where the Hydrogen atoms then slowly insert into the triple bond of the alkyne.
Thus Hydrogenation of alkynes is stereoselective which happens through syn addition. Stereoselectivity leads to formation of an unequal mixture of stereoisomers (isomeric molecules having same molecular formula but different three dimensional orientations of the atoms in space). The reaction is generally exothermic.
- References
- http://en.wikipedia.org/wiki/Lindlar_catalyst
- http://en.wikipedia.org/wiki/Stereoisomerism
- http://en.wikipedia.org/wiki/Syn_and_anti_addition
- https://www.masterorganicchemistry.com/2011/08/19/reagent-friday-lindlars-catalyst/
- http://en.wikipedia.org/wiki/Hydrogenation
- http://www.chemgapedia.de/vsengine/vlu/vsc/en/ch/2/vlu/oxidation_reduktion/red_hydr_lindlar.vlu/Page/vsc/en/ch/2/oc/reaktionen/formale_systematik/oxidation_reduktion/reduktion/addition_wasserstoff/hydrierung_alkine/hydrierung_mit_lindlar/mechanismus1.vscml.html