Methoxide
What is Methoxide?
The simplest alkoxides and organic salts are called Methoxides.
Types Of Methoxide
There are various methoxides in existence. They are:
- Sodium methoxide
- Potassium methoxide
- magnesium methoxide
- Calcium methoxide
- Lithium methoxide
All the above chemicals are called methoxides together. The methoxides can be used in the preparation of bio-diesel. All the methoxide compounds decompose in the presence of water.
Methoxide Composition
Methoxides are mainly organic salts and alkoxides. The organic salts are produced by a reaction between an organic acid (organic compound having acidic properties) and an inorganic base (a base that is composed of an inorganic compound). Alkoxide is the conjugate base of an alcohol. An organic group is bonded to a negatively charged oxygen atom in an alkoxide.
Methoxide Ion
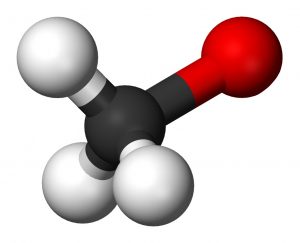
Methoxide ion has the formula of CH3O- in organic chemistry. It means that there are three carbon (C)-hydrogen (H) covalent bonds in a methoxide compound. The carbon atom forms another bond with an oxygen atom that is negatively charged (O-). The methoxide ion is also known as methoxide anion (a negatively charged ion). The ionization of methoxide happens in the following way:
CH3ONa (Sodium methoxide) = CH3O- + Na+
Methoxide is the conjugate base of ethanol (CH3OH). The methoxide ion is extremely strong as a base (substance capable of accepting a hydrogen ion or proton). It is even stronger than a hydroxide ion.
Methoxides should not be kept in the presence of water. This is due to the fact that the methoxide will cause a chemical reaction by removing a proton from the water molecule. It will produce methanol and hydroxide.
Picture 1 – Methoxide Picture
Source – en.wikipedia.org
Sodium Methoxide
Sodium methoxide is a chemical compound. It is most useful among all the methoxides. The chemical formula for sodium methoxide is CH3ONa.
Sodium Methoxide Preparation
Formation of sodium methoxide is the result of the deprotonation of methanol. Methanol is very carefully treated with sodium to prepare sodium methoxide:
2Na + 2 CH3OH → 2 CH3ONa + H2
The resulting solution is colorless and is used as a source for sodium methoxide. To isolate the pure material from the solution, you have to apply heat to it and remove the residual methanol by evaporation.
Properties of Sodium Methoxide
CH3ONa has the following properties:
- Sodium methoxide is a white powder in its normal physical state.
- The melting point of this substance is 127°C.
- The molecular weight of substance is 54.0237 g/mol.
- Its specific gravity is 1.1.
- Its density is 0.99 g/cm3.
Uses of Sodium Methoxide
Sodium methoxide is the most used methoxide. It has the following uses:
- In organic chemistry, CH3ONa is used as a routine base. It is useful in the synthesis of many compounds.
- It is also used in the process of producing methyl ethers as a nucleophile.
- It can be used as an initiator of anionic addition polymerization along with ethylene oxide. It forms a polyether which has a high molecular weight.
- It is widely used to prepare bio-diesel.
Magnesium Methoxide
Magnesium methoxide is a chemical compound. Mg(OCH3)2 is the chemical formula for magnesium methoxide. It is also known as magnesium methylate.
Properties of Magnesium Methoxide
The properties of magnesium methoxide are as follows:
- It is colorless and has a crystallized physical state.
- The crystals of Mg(OCH3)2 decompose if heated.
- The molecular weight of this substance is 86.3728 g/mol.
- It has a density of 0.816 g/cm3.
Uses of Magnesium Methoxide
Read on to know about the various uses of Magnesium methoxide
- It is used as a catalyst.
- It is also used as a dielectric coating and cross-linking agent.
- It is used to form gels.
- It is used to prepare bio-diesel.
Potassium Methoxide
It is yet another methoxide with the chemical formula KCH3O. It is also known as the potassium salt.
Properties of Potassium Methoxide
Potassium methoxide is a compound having the following properties:
- In its basic physical state potassium methoxide has a crystallized form and is white in color.
- KCH3O has the molecular weight of 70.1322 g/mol.
- Its density is 0.85 g/cm3.
Calcium Methoxide
It is a chemical compound having the molecular formula of Ca(OCH3)2. it is also known as calcium salt.
Properties of Calcium Methoxide
The properties of calcium methoxide are as follows:
- It has a solid physical state.
- Ca(OCH3)2 has a molecular weight of 102.15 g/mol.
- The melting point of this substance is 385°C.
This is not a very widely used chemical compound. But it does have its uses at industries.
Lithium Methoxide
Lithium methoxide is another methoxide compound and has the molecular formula of LiCH3O.
Properties of Lithium Methoxide
The properties of lithium methoxide are as stated below:
- It is a colorless or pale yellow liquid.
- LiCH3O is a highly inflammable compound.
- The molecular weight of this substance is 37.97 g/mol.
- The boiling point for LiCH3O is 64.4°C.
- LiCH3O is soluble in methanol.
- It has a density of 0.865 g/cm3.
The methoxide compounds have wide uses in various industries. One of the main uses of the methoxide compounds is in the preparation of bio-diesel. It is very important to know that all the methoxide compounds are highly toxic. Handling them needs special protection.
- References
- http://dictionary.reference.com/browse/methylate
- http://www.home-made-biodiesel.com/methoxide-mixing.html
- https://pubchem.ncbi.nlm.nih.gov/compound/methoxide
- http://www.meta-synthesis.com/chemthes/entity_datapage.php?id=227
- http://chemicalland21.com/industrialchem/organic/POTASSIUM%20METHOXIDE.htm
- http://www.wolframalpha.com/entities/chemicals/lithium_methoxide/tp/io/zs/