Polarizability
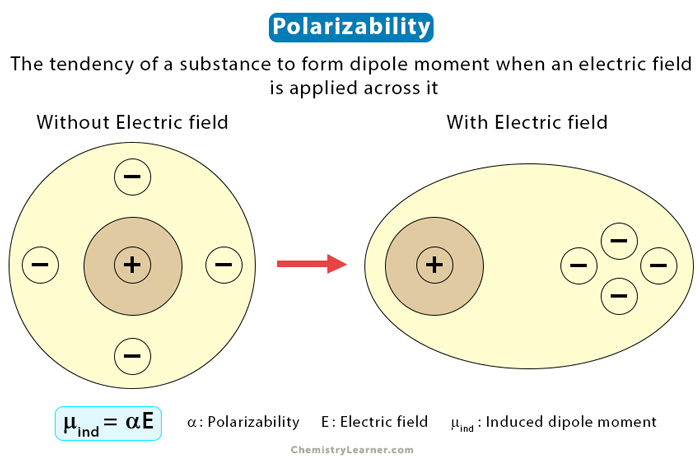
An atom consists of a nucleus with electrons moving around in orbits. When an electric field is applied, the electrons cluster around one part of the atom. When it happens, the ends of the atom become oppositely charged, resulting in an instantaneous dipole. Polarizability refers to the tendency of a substance to form a dipole moment when an electric field is applied across it.
How to Determine Polarizability
The polarizability (α) of a substance is the ratio of the induced dipole moment (μind) to the applied electric field (E). Mathematically, it can be determined from the following equation.
p = αE
The unit of polarizability is C·m2·V-1. It is a scalar quantity meaning that the applied electric field can only produce polarizability components in one direction. It is in the direction of the field.
Factors that Influence Polarizability
1. Number of Electrons
The number of electrons controls how tightly they are bound to the nucleus. Atoms with fewer electrons have a smaller, denser electron cloud with a stronger attraction towards the nucleus. Moreover, the outer electrons will have less shielding from the nucleus, adding to the attractive force. These smaller atoms are generally challenging to polarize. Examples are Mg2+ and Al3+.
On the other hand, large atoms and anions with more electrons have large radii and diffused electron clouds that weaken the nucleus-electron interactions. These atoms and ions can be easily polarized. Examples are I– and Br–.
Trend in the Periodic Table
Since large atoms are easily polarizable, the polarizability trend in the periodic table is similar to that of ionic radius. We know that the ionic radius increases down a group. Hence, the polarizability also increases down a group. The trend across a period is complicated and explained in our article on ionic radius.
2. Molecular Orientation
For unsaturated molecules, like 2,4 hexadiene, the molecular orientation is significant. In these molecules, the polarizability is greatest when the field is applied parallel to the molecule rather than perpendicular.