Resonance Structures
We have learned that Lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. However, a single Lewis structure cannot explain chemical bonding due to partial charges on the atoms and fractional bond order. Sometimes more than one Lewis structure is needed to represent the molecular structure.
Resonance is a technique of describing the delocalized electrons in a molecule or ion that a single Lewis structure cannot describe. It is a phenomenon that explains the shifting of non-bonding electrons and pi bonds within the molecule. As a result of resonance, the molecule can have more than one structure that differs in the delocalized electrons’ position and bond formation. These structures are collectively known as resonance structures. Resonance structures occur in a molecule with multiple ways to place the lone pairs and pi bonds [1-4].
Hybrid Resonance Structures
Even though a molecule displays resonance structures, its actual structure is the weighted average of all the structures. This actual structure is known as the hybrid structure. It represents the overall delocalization of electrons within the molecule. Its energy is lower than any of the resonance structures. Generally, a hybrid structure is slightly different from a dot structure. Dashed lines show the delocalization and fractional bond order [1-8].
Resonance Structures Examples [2,4,7]
1. Ozone (O3)
Ozone has two major resonance structures that contribute equally to its overall hybrid structure. Each oxygen atom has 6 valence electrons, making it a total of 18 for the molecule. 6 out of 18 electrons participate in chemical bonds, and the remaining 12 remain as lone pairs.
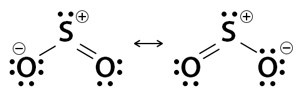
2. Sulfur dioxide (SO2)
Sulfur dioxide has two resonance structures that contribute equally to the molecule’s overall hybrid structure. It flips back and forth rapidly (resonates) between the two forms. The total number of valence electrons is 18 – 6 from sulfur and 6 from each of the two oxygen atoms. 6 electrons form 3 bonds, and 10 electrons distribute themselves as lone pairs on all three atoms.
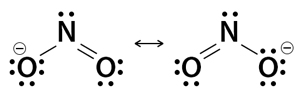
3. Nitrite Ion (NO2–)
There are two resonance structures for the nitrite ion. There are three lone pairs in one oxygen atom, and that oxygen is bonded with the nitrogen atom by a single bond. Also, that oxygen has a -1 charge. The other oxygen atom is bonded to the nitrogen by a double bond and has two lone pairs. Also, there is no charge in that oxygen. There is only one lone pair on the nitrogen atom and no charge on the atom.
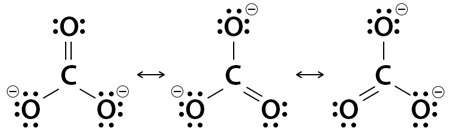
4. Carbonate Ion (CO3-2)
Carbonate ion has 24 electrons, 2 of them responsible for the -2 charge. It has one C-O double bond and two C-O single bonds. Each singly bonded oxygen atom bears a formal charge of ‐1, and all other atoms are neutral.
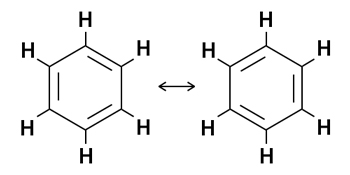
5. Benzene (C6H6)
Benzene has six carbon atoms and a cyclic structure consisting of alternating single and double bonds between adjacent carbon atoms. Each carbon atom is also bonded to one hydrogen atom. Since it has three double bonds, it is expected to be quite reactive. However, it is highly stable. The benzene molecule is stabilized by resonance. The pi electrons are delocalized around the ring structure, resulting in two resonance structures.
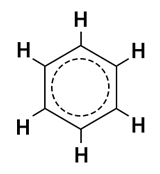
In the hybrid structure of benzene, all the double bonds are replaced by a dashed circle.
The image below summarizes some of the examples discussed above.
How to Draw Resonance Structure [4-8]
Rules of Resonance Structure
In order to draw the resonance structures, one has to keep the following rules in mind:
- They must have the same number of electrons.
- They must be valid Lewis dot structures.
- There is no change in hybridization between the structures.
- Only electrons move through the molecules. The atoms remain fixed in their positions.
- Formal charges of each atom can identify the most significant and contributing resonance structure.
- The structure with the least number of formal charges is more stable than those with more. Neutral structures are more significant than charged structures.
- Structures with complete octet are more significant than incomplete ones.
Based on the above rules, let us try to draw the resonance structures of the nitrate ion (NO3–).
Resonance Structures of Nitrate Ion (NO3–)
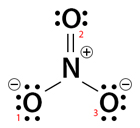
Step 1: Draw the Lewis dot structure of NO3–, as shown below. The procedure is discussed in our Lewis Structure article.
The image shows that NO3– has two oxygen atoms (O1 and O3) with three lone pairs. Also, these two oxygen atoms have a -1 charge and are single bonded to the nitrogen atom. Another oxygen atom (O2) has two lone pairs, is double bonded to nitrogen, and has no charge. The nitrogen has no lone pairs and has a charge +1. The nitrogen and all oxygen atoms obey the octet rule.
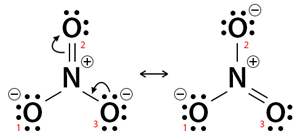
Step 2: Convert one lone pair from a charged oxygen (O3) atom to a bond with nitrogen. The donor atom becomes neutral and forms a new pi bond with nitrogen, which now has 10 electrons in its valence shell. It will avoid violating the octet rule.
However, there is another pi bond between nitrogen and oxygen (O2). This pi bond will break, and the electrons will transfer to the oxygen atom. Nitrogen now has 8 electrons. Since it acquires a lone pair, O2 will have a negative charge. Thus, the negative charge eventually transfers from one oxygen atom (O3) to another (O2). Indicate the electron shift by a curved arrow, as shown in the image below.
Step 3: Step 3 is a repetition of step 2 with the other charged oxygen atom (O1) donating its lone pair to form a pi bond with nitrogen. The process is shown below.
In reality, all three resonance structures do not exist simultaneously. The resonance structures and the resonance hybrid of NO3– are shown below.
Isomer vs. Resonance Structure
Resonance structures are not the same as isomers. Isomers are different compounds having the same molecular formula. Two isomeric compounds can differ by how atoms are bonded. In constitutional isomers, atoms move from one position to another. An example of it is butane and isobutane with the molecular formula C4H10. While butane is a straight-chain molecule, isobutane is a branched-chain molecule. On the other hand, the arrangement of atoms in resonance structures does not change. In other words, the skeleton of the molecular structure remains the same [1].
FAQs
Ans. No, there are no resonance structures of CH2Cl2 since it is polar.
Ans. No. There are no pi bonds in OF2 to display any resonance.
Ans. HCN does not have any major resonance structures. However, it does have an insignificant resonance structure whose contribution is minimal.
Ans. SF4 is a non-polar covalent molecule. Therefore, it does not have any resonance structures.
Ans. SO3 has seven resonance structures.
Ans. All resonance structures are stable and equally contribute to the resonance hybrid in equivalent resonance structures. In non-equivalent resonance structures, one resonance structure is better than the other, and all have different stabilities.