Zinc hydroxide
It is an inorganic chemical compound that naturally exists as a rare mineral. It can dissolve equally in a solution of strong acid or base and is called an amphoteric compound. Under aqueous conditions, the compound dissociates into zinc and hydroxide ions.
Zinc hydroxide Identification
CAS number: 20427-58-1
PubChem: 9812759
ChemSpider: 7988510
Zinc hydroxide Formula
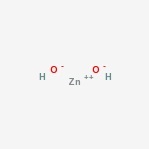
Its chemical formula is Zn (OH)2.
Zinc hydroxide Properties
The following are some of the unique properties of the compound:
Appearance
It generally appears as a white solid.
Molar mass
It weighs around 99.424 g/mol.
Stability
It is stable and non-reactive under normal conditions.
Density
Its mass per volume is around 3.053 g/cm3.
Melting point
It decomposes at 125 degree Celsius.
Solubility
It is sparingly soluble in water and absolutely insoluble in alcohol. Its solubility product (Ksp) is 3.0 x 10-17.
Dissolution
Zinc hydroxide dissolves in aqueous ammonia to form a colorless, water-soluble ammine complex. When ammonia is added in excess, the hydroxide ions that dissociates from Zn(OH)2 reacts with it to form a positively charged tetra complex having a co-ordination number of 4. The resultant product is surrounded by ammonia ligand, which results in dissolution.
Zinc hydroxide Thermo chemistry
Its standard enthalpy of formation or standard heat of formation is -642 kJ.mol-1.
Zinc hydroxide Structure
Here is the structure of the amphoteric compound.
Zinc hydroxide Occurrence
It occurs as three rare earth minerals: Wulfingite, ashoverite and sweetite. These rare minerals are actually the natural polymorphs of Zn (OH)2. In material science, any solid material is polymorphic if it exists in more than one form such as orthorhombic or tetragonal.
Zinc hydroxide Preparation
It is generally prepared by adding moderate amount of sodium hydroxide solution to a zinc salt solution such as zinc chloride or zinc sulfate. These two compounds react under normal conditions to form a white precipitate of Zn (OH)2. Dilute solution of sodium hydroxide should be used in this reaction in order to prevent the inorganic compound from dissolving. Under normal conditions, the zinc salt dissociates to form a zinc ion that associates with two hydroxide ions from sodium hydroxide solution to form zinc hydroxide.
Zn2++ 2OH- → Zn(OH)2
If excess sodium hydroxide is added to the salt solution, the precipitate of Zn (OH)2 that is formed initially will dissolve to form a form a colorless solution of zincate ion as seen below:
Zn (OH)2 + 2OH– → Zn(OH)42-
This property of zinc hydroxide is extensively used to detect the presence of zinc ions in solution. However, it is not a unique method for testing as there many compounds of aluminum and lead that behave in a very similar manner.
The dissolving property of zinc hydroxide can be contributed to the presence of water ligands that normally surround the ion. When excess sodium hydroxide is added to the solution, the two hydroxide ions reduce it to a negatively charged complex, thus making it soluble.
Zinc hydroxide Uses
Some of the common uses of Zn (OH)2 are:
Surgical dressings
It is used for surgical dressings where it functions as an absorbent. Large bandages that are used post surgery are coated with the zinc compound for absorbing the blood from the wound.
Protective coating
Steel and iron are most often applied with a coating of zinc through a process called galvanization. Under moist conditions, a layer of zinc hydroxide forms on these galvanized metals in order to prevent them from getting rusted.
Mordant
Zinc hydroxide is very gelatinous (jelly-like) and generally functions as a mordant that sets dyes on various fabrics or tissues. Dye is generally a chemical reagent, which forms a metal complex with Zn(OH)2 and strongly attaches to the fabric linings.
Pesticides
It is often used as an intermediate for commercial production of pesticides and pigments.
Topical solutions
Combination of Zn (OH)2 with zinc oxide is widely used in calamine creams, ointments, baby powder, skin lotions, and cosmetics. It reacts with strong bases, owing to its amphoteric nature to form zincates that are uses as oral drugs to treat several disorders.
Electrical batteries
It is also used in high-energy electrical batteries that are rechargeable through a reversible reaction.
Rubber compounding
Zn (OH)2 is utilized in compounding or mixing different ingredients of rubber to optimize the end-use properties, according to the consumer’s need.
Zinc compounds
Wide variety of zinc compounds like zinc oxide, zinc sulfate, and zinc nitrate are commercially manufactured with the help of the inorganic chemical.
Zinc hydroxide MSDS
It is generally non-flammable and poses no hazards to the workers. However, the compound should not be tasted or swallowed as it may contain few toxicological ingredients.
- References
- http://en.wikipedia.org/wiki/Zinc_hydroxide
- http://simple.wikipedia.org/wiki/Zinc_hydroxide
- http://www.chemicalbook.com/ChemicalProductProperty_EN_CB5671092.htm

dear sir,
I want to prepared zinc hydroxide from zinc chloride solution but without using sodium hydroxide ,ammonia, and calcium hydroxide pl.guide me which chemical to be used for precipitation of zinc hydroxide
Hi Madhulima, for the precipitation of zinc hydroxide, I don’t which compound should be added during the process. That is my question now. Can you give the simple answer?
Mainly depends up on end uses.normally Potassium hydroxide is common,you may use Mg,or Al.hydroxide.
How can separate CaCl2 from ZnCl2 solution?
I found a Zn liquid fertilizer containing 70% Zn in the form od ZnO and 1.8% Nitrogen in a form of Urea. Normally the solubility constant KSP Zn(OH)2, 3 x 10(-17). I doubt this Zn is not ionized and may not be readily available to plants. Would some one please give me a hint.