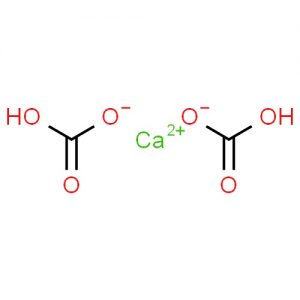
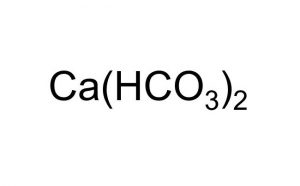Calcium Bicarbonate
Calcium bicarbonate represented by the chemical formula Ca(HCO3)2 or C2H2CaO6 that bears the IUPAC name calcium hydrogen carbonate [1] is a white crystalline powder that is soluble in water [3]. It is an ionic compound of calcium, hydrogen, carbon, and oxygen [1, 6]. Though it is not a naturally occurring compound, it forms in the water of rivers, lakes, and streams when carbonate, bicarbonate, and calcium ions are dissolved with carbon dioxide [5].
Calcium Bicarbonate Identification |
|
| CAS Number | 3983-19-5 [1] |
| PubChem CID | 10176262 [1] |
| ChemSpider ID | 8351767 [2] |
| UNII | 7PRA4BLM2L [1] |
Composition and Synthesis
Calcium bicarbonate can be prepared by a reaction between calcium carbonate and carbonic acid [7].
CaCO3 + H2CO3 = Ca(HCO3)2
Properties and Characteristics of Calcium Bicarbonate
General Properties |
||
| Molar mass/molecular weight | 162.11 g/mol [1] | |
Physical Properties |
||
| Color/appearance | White powder [1] | |
| Melting point/freezing point | 1339°C, 2442°F [3] | |
| Boiling point | N/A [3] | |
| Density | 2.711 g cm-3 [3] | |
| State of matter at room temperature (normal phase) | Solid [1] | |
Chemical Properties |
||
| Solubility in water | 16.6 g/100 ml (at 20oC) [3] | |
| pH | >7 (basic) [4] | |
Atomic Properties |
||
| Crystal structure | Trigonal [3] | |
Prominent Reactions
On heating, calcium bicarbonate decomposes to calcium carbonate, carbon dioxide and water [8].
Ca(HCO3)2 = CaCO3 + CO2 + H2O
It reacts with hydrochloric acid to produce calcium chloride, carbon dioxide and water [9].
Ca(HCO3)2 + 2HCl = CaCl2 + 2CO2 + 2H2O
It reacts with sulfuric acid to produce calcium sulfate, carbon dioxide and water [10].
Ca(HCO3)2 + H2SO4 = CaSO4 + 2CO2 + 2H2O
Calcium bicarbonate reacts with nitric acid to give calcium nitrate, carbon dioxide and water [12].
Ca(HCO3)2 + 2HNO3 = Ca(NO3)2 + 2CO2 + 2H2O
Calcium Bicarbonate Uses
- As a food additive [1].
- As an anti-caking agent [1].
- As a color stabilizer [1].
Is It Dangerous
The compound is not harmful if ingested. However, it may cause irritation to skin, eyes and the respiratory tract on inhalation [3, 5].
Interesting Facts
- Calcium bicarbonate causes temporary hardness of water [13].
- Calcium bicarbonate solution in water has health benefits due to the alkalizing effect and mineral content [5].
Calcium Bicarbonate (Ca(HCO3)2) Price
The compound costs around $200-$245 per ton. The price may vary with suppliers [11].
- References
- Calcium Bicarbonate – Pubchem.ncbi.nlm.nih.gov
- Calcium Bicarbonate – Chemspider.com
- Calcium Bicarbonate – Wikivisually.com
- Calcium Bicarbonate – Sciencedirect.com
- What Is Calcium Bicarbonate? – Livestrong.com
- Why calcium bicarbonate is soluble in water while calcium calcium carbonate is insoluble? – Linkedin.com
- How do you make calcium bicarbonate at home? – Quora.com
- What is the balanced chemical equation for the decomposition of calcium hydrogen carbonate? – Quora.com
- How Do I Balance calcium bicarbonate reacts with hydrochloric acid ? – Socratic.org
- How does calcium carbonate react with sulphuric acid? – Quora.com
- Calcium Bicarbonate Price – Alibaba.com
- How does nitric acid react with calcium carbonate? – Quora.com
- Calcium bicarbonate causes hardness in water but not calcium carbonate. Why – Brainly.in