Single Covalent Bond
What is a Single Covalent Bond?
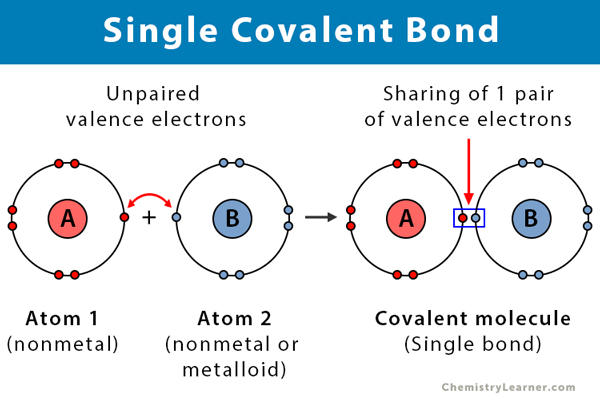
A covalent bond is a type of chemical bond in which the atoms in a compound share electrons. A single covalent bond, or merely a single bond, involves the sharing of one pair of electrons, i.e., two electrons. Atoms combine in order to complete their valence (outermost) shell and become stable. They bond due to strong electrostatic attraction between the bonding electrons and the nuclei of both the atoms. This type of bond is prevalent in alkanes [1-3].
Properties of Single Covalent Bond
- Takes place between two nonmetals or a nonmetal and a metalloid
- Consist of one pair of electrons, i.e., two electrons.
- High bond length
- Stable
Examples of Single Covalent Bond
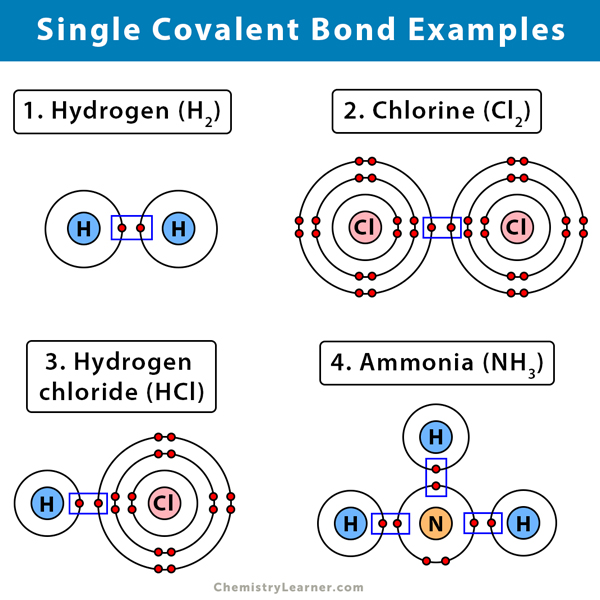
Here are some examples of a single covalent bond [1-3].
1. Hydrogen (H2)
A molecule with a single covalent bond is hydrogen. The hydrogen molecule consists of two hydrogen (H) atoms. Each hydrogen has only one electron. When two atoms of hydrogen combine, the lone electron from each hydrogen takes part in sharing. Thus, a single covalent bond shares two electrons or one pair.
2. Fluorine (F2), Chlorine (Cl2), Bromine (Br2), and Iodine (I2)
Halogens like fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) have seven valence electrons in their outermost shells. Therefore, they need one electron to complete the shells. Two halogen atoms combine and share one electron each. Thus, by sharing one pair of elections, halogens form single covalent bonds.
3. Ammonia (NH3)
Nitrogen (N) has five valence electrons in its outermost shell. It requires three to fill up its orbital. Hydrogen (H), with its lone electron, will combine with nitrogen to form a single covalent bond. Three hydrogen atoms will combine with nitrogen, forming three single covalent bonds.
4. Ethane (C2H6)
An example of an alkane is ethane. Carbon (C) has only four electrons on its outermost shell. It requires four more electrons to complete the shell. Hydrogen (H) has only one electron. Therefore, four hydrogen atoms will share their lone electron with the carbon. The result is four single covalent bonds between carbon and hydrogen.
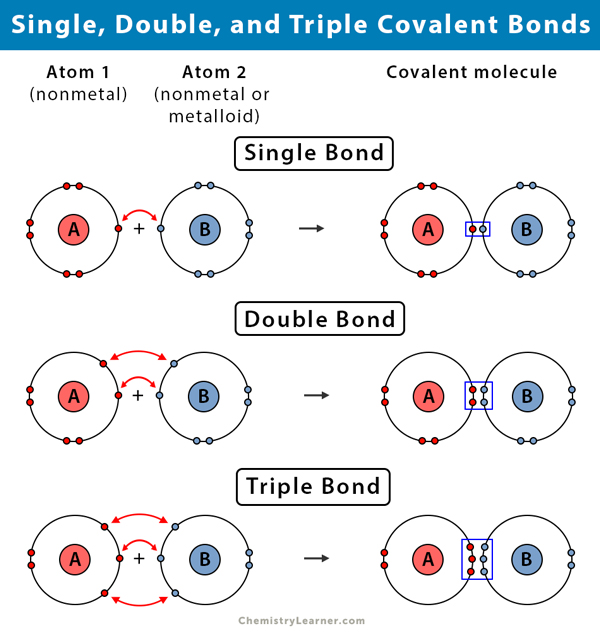
Difference between Single, Double, and Triple Bonds
Single Bond vs. Double Bond vs. Triple Bond | |||
| Single Bond | Double Bond | Triple Bond | |
|---|---|---|---|
|
Number of pairs of shared electrons |
One |
Two |
Three |
|
Bond length |
High |
Moderate |
Low |
|
Bond strength |
Weak |
Strong |
Strong |
|
Reactivity of compounds |
Low (stable) |
Moderate (unstable) |
High (highly unstable) |
|
Denoted by |
Single dash (C-C) |
Double dash (C=C) |
Triple dash (C≡C) |
|
Examples |
Hydrogen (H2), and ammonia (NH3), and ethane (C2H6) |
Oxygen (O2), carbon dioxide (CO2), and ethene (C2H4) |
Nitrogen (N2), cyanide (CN–), and ethyne (C2H2) |