Electroplating
Electroplating, also known as electrodeposition, is the process of depositing one metal onto another through controlled electrolysis. This process results in a thin layer of precious metal coated over the surface of cheap metal. It is mainly used to change the appearance of a substance by making a dull surface look shiny. Many metals like gold, silver, copper, nickel, and chromium are plated this way. Non-metal, like plastic, wood, or glass, that does not conduct any electricity must be made conductive before plating [1-4].
Italian chemist Luigi Valentino Brugnatelli invented electroplating in 1805.
How Does Electroplating Work
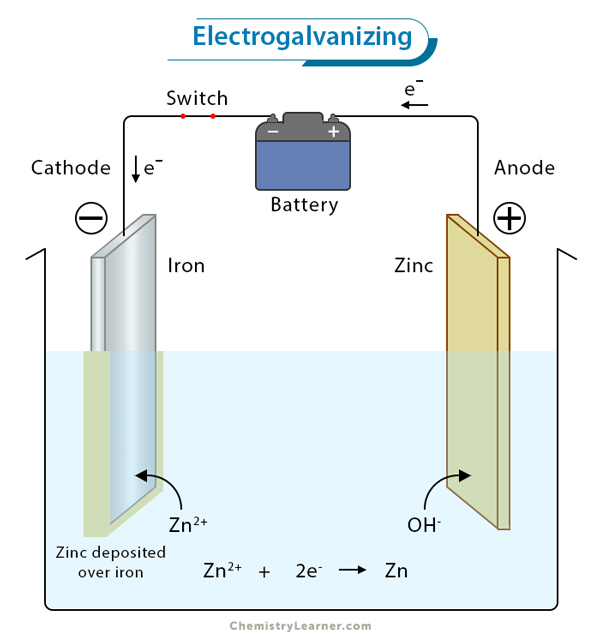
Electroplating works on the principle of electrolysis and takes place in an electrolytic cell. The cell consists of an anode and cathode dipped in an electrolyte. The anode is made of the metal that is to be plated. The cathode is the metal on which deposition takes place. The ends of the two electrodes are connected through a battery. The anode is connected to the positive end, and the cathode is connected to the negative end. The electrolyte is composed of the salt solution of the metal to be deposited [1-6].
When the battery is switched on, current flows through the circuit. Oxidation occurs at the anode, where the metal loses electrons and dissolves in the electrolytic solution. These dissolved metal ions from the electrolyte migrate to the cathode. Reduction occurs at the cathode, where the metal gains electrons and gets deposited. The current passing through the circuit is adjusted so that the anode’s dissolution rate equals the cathode’s plating rate.
Example
Let us look at an example of electroplating. Suppose copper was plated on zinc. In that case, there must be a copper anode, zinc cathode, and a solution of a copper-based compound such as copper sulfate.
When the battery is switched on, electrodes are charged up. Positively charged copper ions are attracted to the negatively charged cathode. They migrate and deposit over zinc, resulting in a thin layer. On the other hand, the negatively charged sulfate ions move to the positively charged anode and release their electrons. These electrons then travel through the wire and reach the cathode.
Equations
The redox reaction taking place is shown below:
Oxidation at anode: Cu (s) → Cu2+ (aq.) + 2e–
Reduction at cathode: Cu+ (aq.) + 2e–→ Cu (s)
Factors Affecting Electroplating [7]
Electroplating of metals can take time depending upon several factors. These factors include:
- the strength of electric current
- the concentration of the electrolyte
- the surface area of the electrodes
- temperature
- the distance between the cathode and the anode
Increasing the current or concentration increases the speed at which the ions move and hence, the deposition process.
Applications [1]
It is used to:
- ornament cheap metals like iron with precious metals such as gold, silver, nickel, and chromium
- give a shiny appearance to everyday objects like keys, chains, jewelry, badges, utensils, and medals with a wide range of decorative finishes
- prevent heat and rust, improve wear resistance, and protect parts of an automobile
- make an airplane’s body resistant to environmental conditions
- increase electrical conductivity and prevent overheating of electronic parts in computers and smartphones
- increase the thickness of surfaces