Elementary Reaction
What is an Elementary Reaction [1-2]
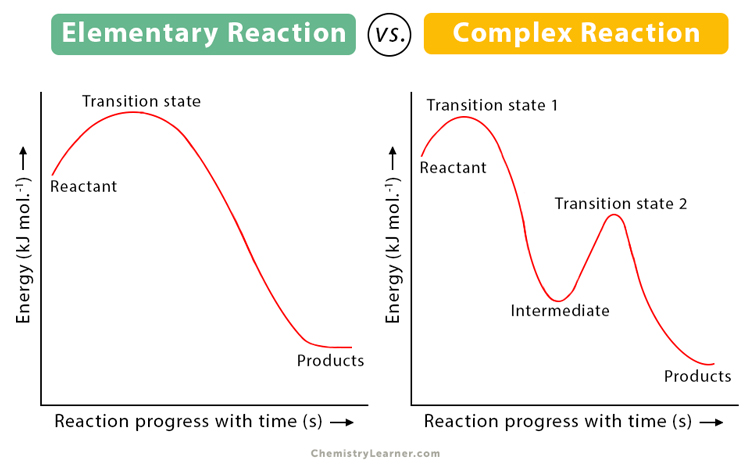
An elementary reaction is a chemical reaction where reactants directly form products in a single step with a single transition state. Here, no intermediate products are formed.
The transition state is the phase of the reaction where the reaction attains the highest energy. It is a very short-lived stage, where the original chemical bonds of the reactants are broken down to form new ones.
Example: Hydrogen iodide (HI) decomposes into hydrogen (H2) and iodine (I2) in a single step.
2HI → H2 + I2 (equation 1)
Molecularity and Rate Law of Elementary Reaction [1-4]
The molecularity of an elementary reaction can be defined as the number of reactants involved in the reaction. It is a determining factor for the classification of elementary reactions. For instance, the reaction given in equation 1 involves 2 molecules of HI. So, its molecularity is 2.
The rate law for an elementary reaction gives a relationship between the reaction rate and the reactants’ concentrations. It can be expressed in terms of the concentration and stoichiometric coefficients of the reactants in the balanced equation. The stoichiometric coefficient is the number present in front of the reactant. For example, the rate law for equation 1 is given by: rate = k[HI]². Here, [HI] is the concentration of hydrogen iodide.
The number of reactant molecules can determine the order of a chemical reaction. It is obtained by taking the sum of all the stoichiometric coefficients of each reactant. For an elementary reaction, the overall reaction order is always equal to molecularity. Again, going back to equation 1, the order is 2, as 2 molecules of HI are taking part in the reaction. Therefore, the decomposition of HI is a second-order reaction.
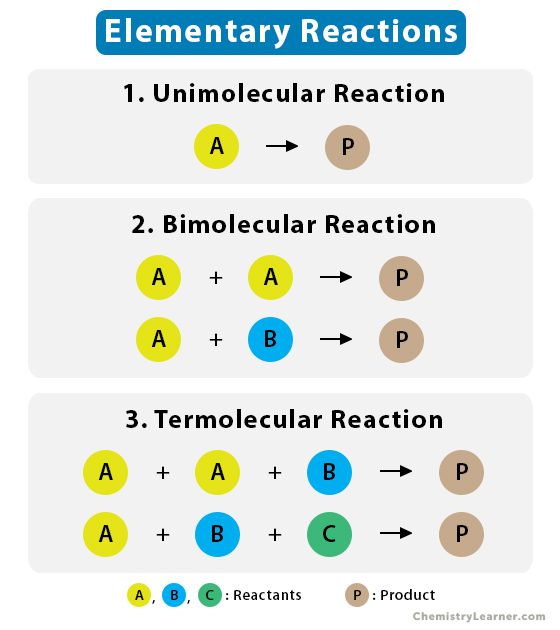
1. Unimolecular Reaction
A unimolecular reaction is an elementary reaction that involves just one reactant molecule, which gets decomposed or rearranged.
General Reaction
A → products
Rate Law: Rate = k[A]
Where [A] is the concentration of A in moles per liter.
Order: First
Example: The gas-phase decomposition of cyclobutane (C4H8) to ethylene (C2H4) occurs via a unimolecular, single-step mechanism.
C4H8 → 2 C2H4
∴ Rate = k [C4H8]
2. Bimolecular Reaction
A bimolecular reaction is an elementary reaction involving two reactants.
General Reaction
A + A → Products Rate = k[A]2
A + B → Products Rate = k[A][B]
Order: Second
Example: The reaction between nitrogen dioxide (NO2) and carbon monoxide (CO) gives nitric oxide (NO) and carbon dioxide (CO2).
NO2(g) + CO(g) → NO(g) + CO2(g)
∴ Rate = k [NO2][CO]
3. Termolecular Reaction
An elementary termolecular reaction involves the simultaneous collision of three atoms, molecules, or ions. A termolecular reaction involves the reaction between three atoms, molecules, or ions. Some termolecular reactions exist but are rare. It is difficult for three atoms or molecules to react by colliding with proper orientation during a chemical reaction.
General Reaction
2A + B → products Rate = k[A]2[B]
A + B + C → products Rate = k[A][B][C]
These are third-order reactions.
Example: The reaction of nitric oxide (NO) with chlorine (Cl) gives nitrosyl chloride (NOCl).
2NO + Cl2 -> 2NOCl
∴ Rate = k [NO]2[Cl2]
Elementary and Non-elementary Reaction [1]
In an elementary reaction, reactants directly form the products without any intermediates. In contrast, in a non-elementary or complex reaction, reaction intermediates form the final products. Also, two or more elementary reactions combine to form a non-elementary reaction.
Here is an example of a non-elementary reaction:
Nitrogen dioxide (NO2) reacts with fluorine (F2) in two steps, producing nitryl fluoride (NO2F).
Step 1: NO2 + F2 → [NO2F + F]* (Slow)
Step 2: [NO2 + F]* → NO2F (Fast)
The overall reaction is:
2NO2 + F2 → 2NO2F
Here, [NO2 + F]* is the intermediate. It is represented by []* to indicate that the intermediate is unstable and short-lived.
| Features | Elementary Reaction | Non-elementary or Complex Reaction |
|---|---|---|
| Definition | A type of chemical reaction involving a single step | A type of chemical reaction involving multiple steps |
| No. of transition states | One | More than one |
| Intermediates | Cannot be detected | Can be detected |
| Nature | Simple | Complex |
| Order | i. Must be an integer ii. Equal to the sum of stoichiometric coefficients of reactants | i. Maybe an integer or a fraction ii. Derived from the slowest step of the overall reaction |
References
- Elementary Reaction Definition – Thoughtco.com
- Reaction Mechanisms – Opentextbc.ca
- Reaction Mechanisms – Chem.libretexts.org
- Elementary Reactions – Chem.libretexts.org