Substitution Reaction
Definition: What is a Substitution Reaction?
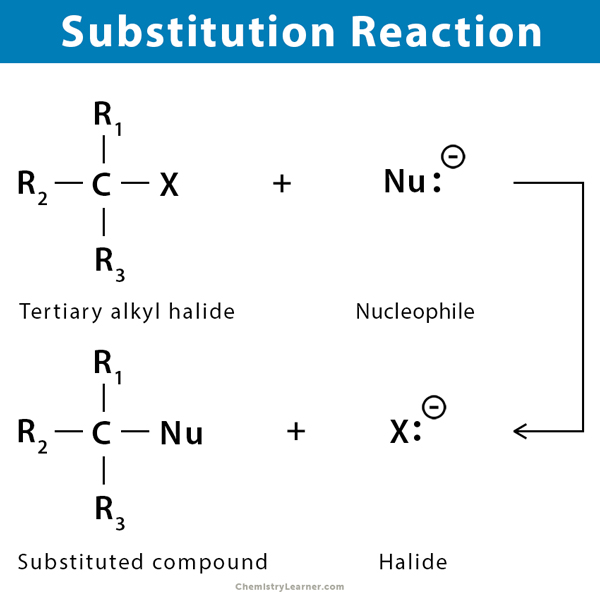
A substitution reaction is an organic chemical reaction during which a functional group replaces an atom or another functional group attached to a carbon atom in a compound.
Components of a Substitution Reaction
An electron-rich species donates a pair of electrons to an electron-poor species and forms a new product and a new base. Therefore, a substitution reaction contains four components.
- Nucleophile: the electron-rich species donating a pair of electrons to carbon
- Electrophile: the electron-deficient species accepting a pair of electrons
- Product: the species that is formed from a substitution reaction
- Leaving group: the group that leaves the compound
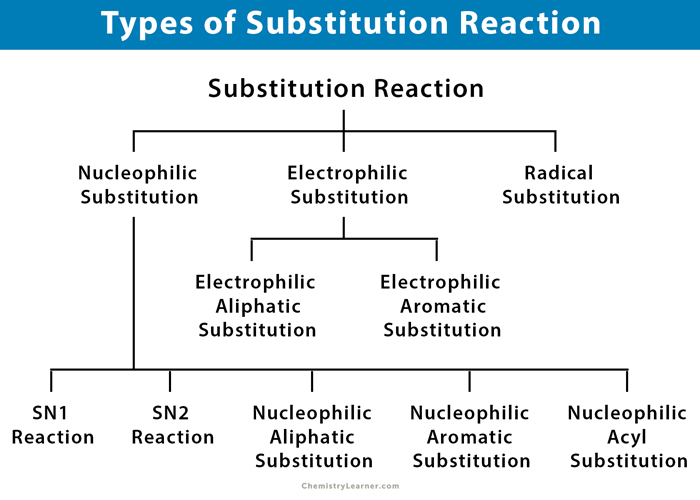
Types of Substitution Reaction
There are three general classes of substitution reactions, depending on the following factors.
- Reactant or substituent
- Intermediate – carbocation, carbanion, or free radical
- Substrate (compound) – aliphatic or aromatic
1. Nucleophilic Substitution
When electron-rich species (nucleophile) provides an electron pair for bonding with the compound being transformed, it is called nucleophilic substitution. Some examples of nucleophiles are Cl–, Br–, I–, OH–, RO–, CN–, H2O, and NH3.
Types of Nucleophilic Substitution:
- SN1 reaction
- SN2 reaction
- Nucleophilic aliphatic substitution
- Nucleophilic aromatic substitution
- Nucleophilic acyl substitution
2. Electrophilic Substitution
When the substituent is electron-deficient (electrophile) and accepts an electron pair for bonding with the compound to be transformed, it is called electrophilic substitution. Some examples of electrophiles are H3O+, NO2+, and SO3.
Types of Electrophilic Substitution:
- Electrophilic aliphatic substitution
- Electrophilic aromatic substitution
3. Radical Substitution
Radical substitution takes place when an atom or group of atoms in a molecule is replaced by a free radical. Some examples of free radicals are Br, Cl, I, and OH.
Applications of Radical Substitution:
- Barton-McCombie deoxygenation
- Wohl-Ziegler reaction
- Hunsdiecker reaction
- Dowd-Beckwith reaction
- Barton reaction
- Minisci reaction
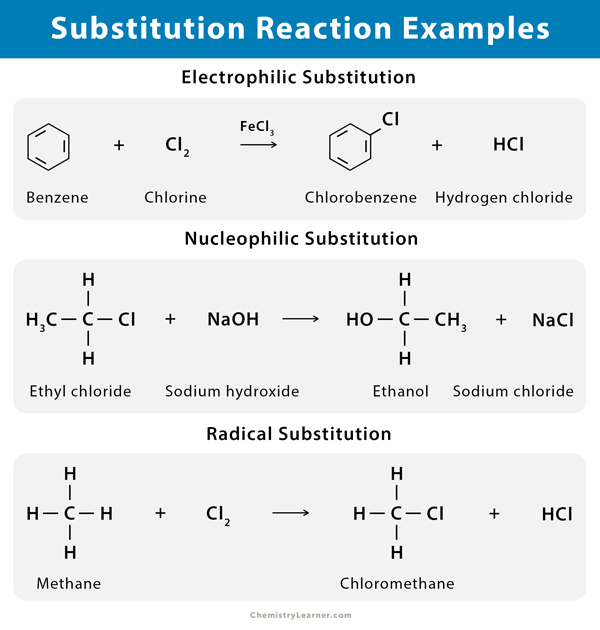
Examples of Substitution Reaction
Halogenation reactions are widespread examples of substitution reactions. Alkane can react with a halogen gas in the presence of ultraviolet light giving alkyl halide. Examples include benzene and methane undergoing chlorination resulting in chlorobenzene and chloromethane (methyl chloride), respectively. Displacement of a good leaving group on an aromatic ring by a nucleophile is an example of nucleophilic aromatic substitution.
Mechanism of Substitution Reaction
Each type of substitution reaction has a unique mechanism. There is no general way to represent them.
Applications of Substitution Reaction
The following organic reactions use the mechanism of substitution reaction.
- Williamson ether synthesis
- Friedel-Crafts acylation
- Friedel-Crafts alkylation
- Halogenation
- Fischer esterification
- Mitsunobu Reaction
- Haloform Reaction
- Sandmeyer Reaction





Easier for students to understand
Thanks for sharing
Well organized and understandable
Good explanation and understanding the content through simply way. Thank you
Hi abdullahi please to verify application of substituation reaction
Thank you????