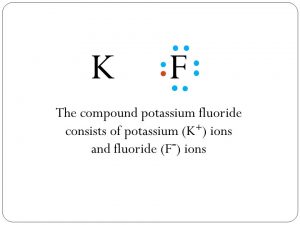
Potassium Fluoride
Potassium fluoride, represented by the chemical formula KF, is an inorganic compound comprising an alkali metal potassium and monoatomic anion fluoride [1]. It exists in its solid state or aqueous solution form, with the mineral carobbiite being the naturally occurring KF [1]. It also exists in other compounds like potassium fluoride dihydrate (KFH4O2) and potassium fluoride tetrahydrate (KFH8O4) [1].
How is Potassium Fluoride Prepared
It is synthesized by reacting potassium carbonate with excess hydrofluoric acid and then evaporating the subsequent solution to form potassium bifluoride crystals [4]. The crystals are then heated to produce potassium fluoride, as shown by the following equations [4]:
K2CO3 + 4HF → 2KHF2 + H2O + CO2↑
KHF2 → KF + HF↑
Reactions with Other Compounds
Silver Nitrate and Potassium Fluoride
If an aqueous solution of potassium fluoride is mixed with a solution of silver nitrate, a double displacement reaction occurs producing silver fluoride, as shown below [5]:
2KF + 2AgNO3 → 2KNO3 + 2AgF
Potassium Fluoride and Hydrobromic Acid
When potassium fluoride reacts with hydrobromic acid, it undergoes a double displacement reaction to form potassium bromide and hydrofluoric acid [6]:
KF + HBr → KBr + HF
Potassium Fluoride and Water
Reacting potassium fluoride with water causes it to completely dissociate wherein the fluorine ion mixes with water to produce hydrofluoric acid, and the potassium ions remain inactive as spectator ions. The ionic equation is as follows [7]:
F– + H2O ↔ HF + OH–
Properties and Characteristics of Potassium Fluoride
General Properties |
|
| Molar Mass/Molecular Weight | 58.097 g/mol (anhydrous) [1, 3] |
Physical Properties |
|
| Color and Appearance | White/colorless deliquescent, crystalline powder [1] |
| Melting Point | 858 °C, 1576 °F (anhydrous) [1, 3] |
| Boiling Point | 1502 °C, 2741 °F [1] |
| Density | 2.48 g cm-3 [1, 3] |
| State of matter at room temperature | Solid [1, 3] |
| pH | 8.5-10.3 at 25 °C [9] |
| Solubility | Insoluble in alcohol but soluble in hydrogen fluoride [1] |
| Solubility in Water | 102 g/100 ml at 25 °C, 92 g/100 ml at 18 °C [1] |
| Magnetic Susceptibility (χ) | -23.6 X 10-6 cm3 mol-1 [8] |
Atomic Properties |
|
| Crystal Structure | Cubic |
What Is It Used for
- Salt fluoridation as a means of community-based fluoridation through the addition of KF to iodized salt, with fluoride concentration less than 250 mg per kg [1].
- Preparing silver soldering flux used by crafters and metal workers [1].
- Increasing the rate of chemical reactions as a catalyst in organic synthesis [1].
- Etching and frosting glass because of the synthesis of soluble fluorosilicates [1].
- Making disinfectants, insecticides, and pesticides [1].
Is It Safe
Ingestion or inhalation of excessive amounts of KF can cause acute toxicity [1]. Being highly corrosive, its contact with skin can result in severe burns [1]. Although it is non-combustible, it decomposes upon heating to yield toxic fumes [1].
References
- Potassium Fluoride – Pubchem.ncbi.nlm.nih.gov
- Potassium Fluoride – Chemspider.com
- Potassium Fluoride – Americanelements.com
- Potassium Carbonate React with Hydrogen Fluoride – Chemiday.com
- Precipitation Reactions – Chem.libretexts.org
- Chemical Equation Balancer – En.intl.chemicalaid.com
- Thermodynamic properties and solubilityof potassium fluoride in aqueous solutions at various temperatures – Sciencedirect.com
- Magnetic Susceptibility Of The Elements And Inorganic Compounds – Gemstonemagnetism.com
- Potassium Fluoride – Hamptonresearch.com


l would like to be a part of this learning era