Single-replacement Reaction
- What is a Single-replacement Reaction?
- What Happens in a Single-replacement Reaction?
- Examples of Single-replacement Reaction
- How to Balance Single-replacement Reaction?
- Rules for Prediction of Single-replacement Reaction
- Types of Single-replacement Reaction
- Single-replacement Reactions Examples in Everyday Life
What is a Single-replacement Reaction?
A single-replacement reaction, also called a single-displacement reaction, is a reaction in which one element is substituted for another element in a compound. In order to understand the chemistry of a single-replacement reaction, one has to know about the activity series of elements in the periodic table. Elements that are higher in the series have a greater tendency to gain or lose electrons and hence, can easily displace elements that are lower in the series. Both metals and nonmetals participate in replacement reactions [1-4].
General Equation for Single-replacement Reaction
The single-displacement reaction equation is:
A + BC → AC + B
This reaction will proceed if A is more reactive than B. The reaction must be balanced when writing an actual reaction [1].
What Happens in a Single-replacement Reaction?
A characteristic of a single-replacement reaction is that one cation or anion trades places with another to form a new product. Metals that are more reactive than hydrogen readily dissolve in water. They form hydroxides and release hydrogen gas.
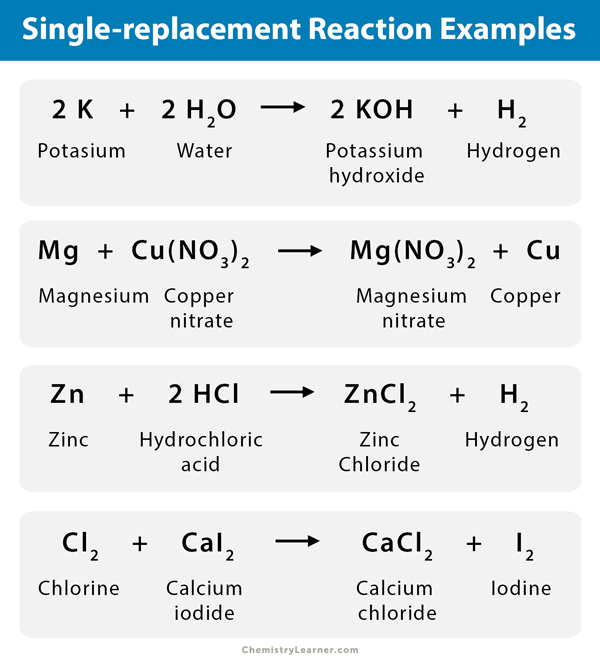
Examples of Single-replacement Reaction
An example of a single-displacement reaction occurs when potassium (K) reacts with water (H2O). A colorless solid compound named potassium hydroxide (KOH) forms and hydrogen gas (H2) is set free. The equation for the reaction is:
2 K (s) + 2 H2O (l) → 2 KOH + H2 (g)
How to Balance Single-replacement Reaction?
Consider the following example. Magnesium (Mg) replaces hydrogen (H) in hydrochloric acid (HCl) and forms magnesium chloride (MgCl2) and gaseous hydrogen (H2).
Mg (s) + HCl (aq.) → MgCl2 (s) + H2 (g)
This equation is not balanced. We note that two hydrogen and chlorine atoms are on the right-hand side of the equation. So, we multiply the compound HCl on the left by 2 and balance the two atoms.
Mg (s) + 2 HCl (aq.) → MgCl2 (s) + H2 (g)
Rules for Prediction of Single-replacement Reaction
The periodic table or an activity series can help to predict whether single-replacement reactions occur. The relative reactivities of the two elements can determine whether one element will replace another element from a compound. A single-replacement reaction will occur when a less reactive element can be replaced by a more reactive element in a compound [2].
1. Halogens
Order of reactivity of the halogens:
More reactive F2 > Cl2 > Br2 > I2 Less reactive
From this order, one can see that chlorine (Cl2) will replace bromine (Br2) from a bromide compound but cannot replace fluorine (F2) from a fluoride compound.
2. Metals
Order of reactivity of metals:
More reactive Cu > Ag > Hg > Pt > Au Less reactive
This order shows that copper (Cu) will replace silver (Ag) in an aqueous solution consisting of Ag+ ions but not vice-versa.
Types of Single-replacement Reaction
There are two types of single-replacement reactions [2-4].
1. Cation Replacement
A cation is a positively charged ion or a metal. In this type of reaction, one cation replaces another.
Examples
- Zinc (Zn) can safely dissolve in hydrochloric acid (HCl), releasing hydrogen (H2) gas [1].
Zn (s) + 2 HCl (aq.) → ZnCl2 (s) + H2 (g)
- Copper (Cu) can displace silver (Ag) in an aqueous solution of silver nitrate (AgNO3).
Cu (s) + 2 AgNO3 (aq.) → Cu(NO3)2 (aq.) + 2 Ag (s/ppt.)
2. Anion Replacement
An anion is a negatively charged ion or a nonmetal. In this type of reaction, one anion replaces another.
Examples
- Chlorine (Cl2) will displace the bromine (Br2) in a sodium bromide (NaBr) compound because it is more reactive than bromine [1].
Cl2 (aq.) + 2 NaBr (aq.) → 2 NaCl (aq.) + Br2 (aq.)
- Bromine (Br2) replaces iodine (I2) in a reaction between bromine (Br2) and potassium iodide (KI).
Br2 (aq.) + 2 KI (aq.) → 2 KBr (aq.) + I2 (aq.)
Single-replacement Reactions Examples in Everyday Life
A few examples of single-replacement reactions occur in nature and real life.
- The reaction of saltwater with concrete pillars containing iron forms iron (II) chloride
- Silver reacts with hydrogen sulfide gas produced by some industrial processes or from decaying animal or plant materials. The reaction results in silver sulfide and hydrogen gas.