Aryl Halide
An aryl halide is an organic molecule in which one or more halogen atoms are bonded to a benzene ring or any other aromatic ring. The aryl group is a functional group devoid of a hydrogen atom. We get an aryl halide when an aryl group combines with a halogen atom. [1-4]
Aryl halides exhibit unique reactivity patterns and have played a crucial role in the development of modern chemical synthesis. They are an essential class of compounds in medicinal chemistry. Moreover, the aromatic ring imparts remarkable stability to these compounds due to the delocalization of electrons in a conjugated π-electron system.
Structure and Nomenclature
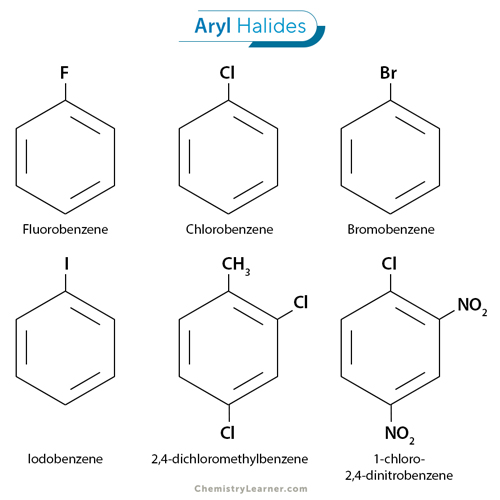
Aryl halides belong to the larger class of organohalides, which are organic compounds containing carbon-halogen (C-X) bonds. The general structure of an aryl halide is a benzene ring in which a hydrogen atom is substituted by a halogen such as fluorine (F), chlorine (Cl), bromine (Br), and iodine I). [1-4]
The nomenclature of aryl halides follows the same conventions as other organic compounds, with the halogen names as a substituent attached to the aromatic ring. For instance, a compound containing a chlorine atom attached to a benzene ring is called chlorobenzene. Other examples of aryl halide include fluorobenzene, bromobenzene, and iodobenzene. Sometimes more than one hydrogen atom is substituted. An example of such a compound is 2,4-dichloromethylbenzene.
Synthesis
Several methods are available for synthesizing aryl halides, each catering to specific needs and reactions. Some common synthetic routes include: [1-4]
1. Halogenation of Arenes
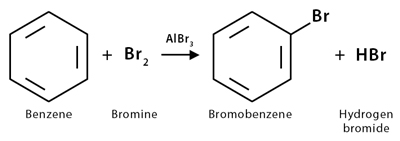
The most common way of preparing an aryl halide is by halogenation. Halogenation is a chemical reaction in which a halogen is added to a hydrocarbon by replacing one of its hydrogens. Here, an electrophilic halogenating agent such as Cl2 or Br2 replaces hydrogen in an arene. This kind of reaction is known as electrophilic aromatic substitution.
2. Sandmeyer Reaction
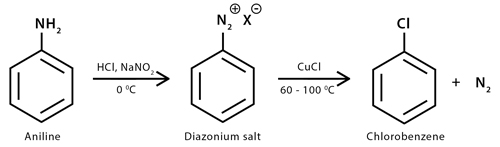
A second method of preparation is through Sandmeyer reaction. It is a radical-nucleophilic aromatic substitution reaction. It is a helpful tool by which an amino group on an aromatic ring is replaced with a halide. During the Sandmeyer reaction, the amino group is converted into a diazonium salt that can be transformed into a halogen group using copper (I) halides as reagents.
Properties
Here are some distinctive properties of aryl halides. [1-4]
1. Aromaticity and Stability
Aryl halides possess aromatic rings, which exhibit high stability due to their delocalized π-electron system. This exceptional stability of these compounds makes them less reactive than their non-aromatic counterparts. The halogen atoms attached to the aromatic ring can influence the overall aromaticity and stability, with some halogens having electron-withdrawing effects that can destabilize the aromatic system.
2. Polarity and Reactivity
The electronegativity of the halogen atoms contributes to the polarity of aryl halides. The polarity arises from the difference in electronegativity between the carbon-halogen bond. This polarity influences their reactivity, making aryl halides useful in various synthetic reactions, such as nucleophilic aromatic substitution (SNAr) and cross-coupling reactions.
Reactivity and Chemical Reactions
The reactivity of aryl halides is governed by both the nature of the halogen atom and the substituents on the aromatic ring. The halogen atom’s electronegativity influences the compound’s reactivity toward nucleophiles and bases. Aryl halides partake in the following chemical reactions. [1-4]
1. Nucleophilic Aromatic Substitution
A nucleophile replaces a halogen atom on the aromatic ring in this reaction. The reaction rate is influenced by factors such as the nature of the halogen atom, the nature of the nucleophile, and the substituents on the aromatic ring. Aryl halides with electron-withdrawing groups tend to undergo SNAr more readily.
Example
1-chloro-4-nitrobenzene (Cl-(C6H4)-NO2) reacts with sodium methoxide (CH3ONa) in presence of methanol to produce 1-methoxy-4-nitrobenzene (CH3O-(C6H4)-NO2).
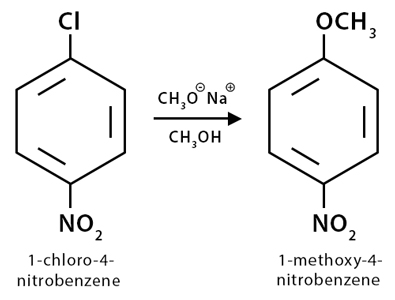
The mechanism for this reaction is called the addition-elimination mechanism. A detailed description is discussed here.
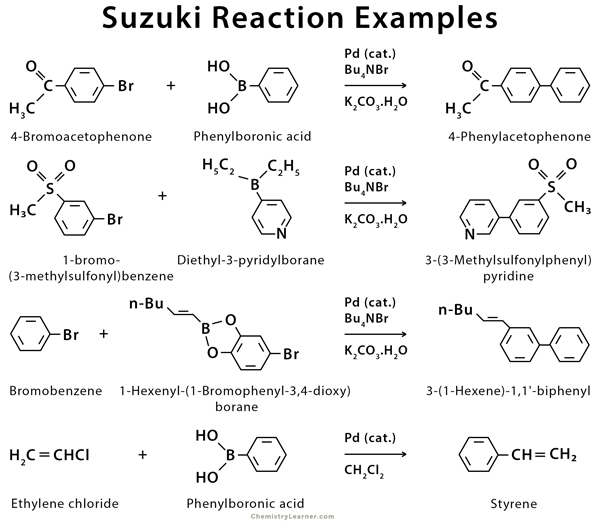
2. Cross-Coupling Reaction
Aryl halides are frequently utilized in cross-coupling reactions, which involve the formation of a new carbon-carbon bond between two different aryl or alkyl groups. Palladium-catalyzed reactions, such as the Suzuki-Miyaura and Heck reactions, are examples of cross-coupling reactions employing aryl halides. These reactions significantly affect synthesizing complex organic molecules, including pharmaceuticals and materials.
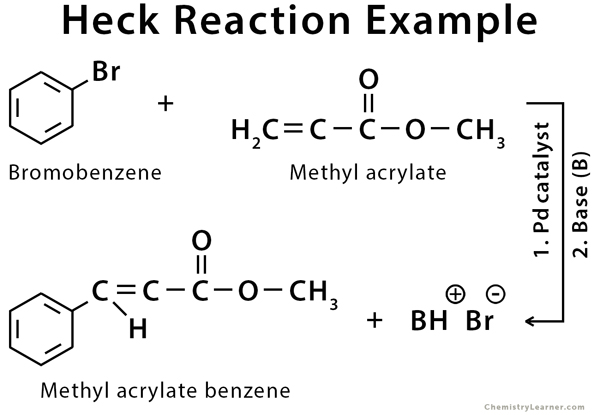
3. Preparation of Grignard Reagent
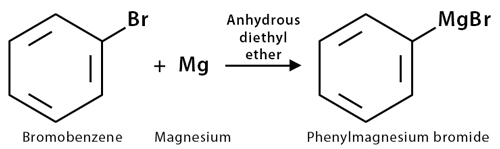
Grignard reagent is an organomagnesium compound formed by the reaction of an aryl halide with magnesium metal via a radical mechanism. For example, phenylmagnesium bromide is prepared by the reaction of bromobenzene with magnesium metal. Grignard reagent reaction is a powerful method for forming carbon-carbon bonds and constructing complex molecules.