Chemical Reactivity
Chemical reactivity measures how readily a substance undergoes a chemical reaction by itself or by reacting with another substance. From a thermodynamic point of view, a chemical reaction occurs when the products have lower free energy than the reactants. The reaction is accompanied by a release of energy, allowing the products to reach a stable state. Reactivity is a chemical property of a substance [1-4].
Chemical reactivity may refer to
- the chemical reactions of a single substance (for example, decomposition)
- the chemical reaction of a substance reacting with another substance (see examples below)
- the methodical study of a set of reactions of the above two types
- the methods used to observe all the chemical reactions experimentally
- the theories that predict chemical reactions
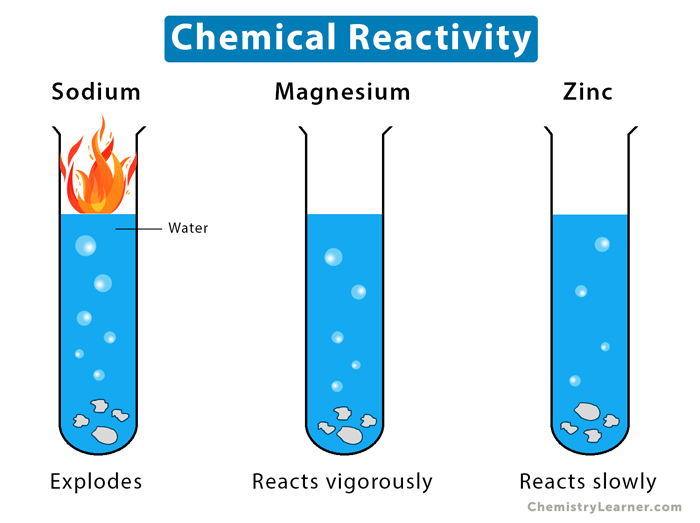
The image below shows metals’ reactivity to water.
Examples [1-4]
- Volatile substances like sodium and potassium ignite in the presence of oxygen, releasing massive energy.
- Baking soda quickly reacts with vinegar producing carbon dioxide and aqueous sodium acetate.
- Iron reacts with oxygen in the environment to form a reddish-colored substance called rust, whose chemical formula is Fe2O3.xH2O.
How Reactivity Works
The reactivity of an atom arises from the existence of unpaired electrons in the valence shell of that atom. Let us look into valence bond theory to understand how atoms achieve stability during chemical bonding. We know that a set of quantum numbers designates an electron. Keeping the principle and azimuthal quantum numbers constant, the order of stability of electrons from least to greatest is as follows: [1-4]
unpaired electrons without any other electrons in the same sublevel < unpaired with all degenerate orbitals of the same sublevel half-filled < filled set of orbitals
A simple explanation for reactivity is that it increases with the ease of donating or accepting electrons. An atom reacts with another atom and forms a bond, resulting in the stability of the individual atoms. Let us look at a few examples to illustrate this point.
Hydrogen (H2)
A hydrogen atom has only one electron in its 1s orbital. It will react with another hydrogen atom to form a hydrogen molecule. The two unpaired electron forms a stable bonding pair.
Carbon (C)
A carbon atom has 4 electrons with ground state valence electron configuration of 2s2 2p2, which is half filled. However, going from the half-filled to completely filled p sublevel requires low activation energy. The result is that carbon forms bonds immediately (sp3 hybridization) while releasing a large amount of energy.
Reactivity and Rate Law
Reactivity can also define how fast substances are reacting. If reactant A produces product B, then the rate of reaction is given by [1-4]
Rate = k[A]
Where
Rate: Change in molecular concentration of a substance in the rate-determining step of the reaction
[A]: Concentration of the reactant A
k: Reaction constant
The greater the reactivity of a substance, the higher the reaction constant. Therefore, higher reactivity results in a higher rate.
Factors Affecting Reactivity [1-4]
1. Temperature
The temperature of a reaction plays a crucial role in chemical reactivity. Increasing the temperature gives the substance the energy necessary to overcome the activation energy barrier and proceed with the reaction.
2. Nuclear Charge
High nuclear charge results in a strong attraction between the outermost electrons and the nucleus. As a result, the atom reduces in size, and the electrons are held tightly. A metal will find it challenging to form chemical bonds with other atoms. Hence, the reactivity decreases as the nuclear size increases.
3. Electron Shielding
The presence of inner electrons shields the outer electrons from interacting with the nucleus. As a result, during covalent bonding, the shared electron pair is not tightly bound to the nucleus. Thus, the reactivity of metals decreases as the shielding increases.
I know much about chemical properties now because this apparent explanation. I will be delighted if you can help me with the note of chemical properties, a good one