Silver Sulfide
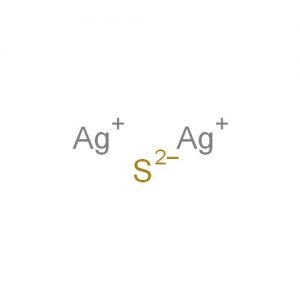
Silver sulfide represented by the chemical formula Ag2S [1] is a black crystalline powder that is soluble in nitric acid, sulfuric acid and alkali cyanide solutions. Ag2S is insoluble in alcohol [1, 5]. It occurs in nature as the mineral argentite and is an ionic compound [5, 6]. It is very stable and doesn’t decompose easily except at an extremely high temperature or by the electrolysis of the molten compound [11].
Silver Sulfide Identification |
|
| CAS Number | 21548-73-2 [1] |
| PubChem CID | 30686 [2] |
| ChemSpider ID | 145878 [3] |
| EC Number | 244-438-2 [1] |
Composition and Synthesis
Silver sulfide can be prepared by a reaction between silver and gaseous hydrogen sulfide. Hydrogen gas is released in the process.
2Ag + H2S = Ag2S + H2
Our silverware tarnishes because of the above reaction. Air contains traces of hydrogen sulfide which causes the silver to tarnish forming silver sulfide. You can polish your silver utensils clean by rubbing them with a cloth and polishing compounds [7] It can be represented by the below reaction with aluminum that involves the reduction of silver sulfide to silver [13].
3Ag2S + 2Al = 6Ag + Al2S3
Properties and Characteristics of Silver Sulfide
General Properties |
||
| Molar mass/molecular weight | 248.804 g/mol [2] | |
Physical Properties |
||
| Color/appearance | Black powder [1] | |
| Melting point/freezing point | 845°C, 1553°F [1] | |
| Boiling point | N/A [1] | |
| Density | 7.234 g cm-3 [1] | |
| State of matter at room temperature (normal phase) | Solid [1] | |
| Resistivity | 0.1 – 10 ohm-m [14] | |
Chemical Properties |
||
| Solubility in water | Insoluble [5] | |
| pH | <7 (acidic) [6] | |
Atomic Properties |
||
| Crystal structure | Orthogonal [4] | |
| Band gap of nanoparticles | 0.96 eV [10] | |
Prominent Reactions
Silver sulfide reacts with hydrochloric acid to produce silver chloride and hydrogen sulfide [9].
Ag2S + 2HCl = 2AgCl + H2S
It reacts with nitric acid to form silver sulfate, nitric oxide, and water [8].
3Ag2S + 8HNO3 = 3Ag2SO4 + 8NO + 4H2O
Silver Sulfide Uses
- The compound finds application in anti-microbial and anti-bacterial agents [4].
- As a photosensitizer in photography [4].
- As a lab reagent [4].
- Inlaying in niello metalwork and ceramics [5].
Is It Dangerous
The compound is irritating to the eyes, skin, and respiratory system. Hence contact and inhalation should be avoided [4]. Ingestion is harmful as it may cause health hazards like irreversible pigmentation during metabolism [12].
Silver Sulfide (Ag2S) Price
5 grams of the compound costs around $80 [8].
- References
- Silver Sulfide – Americanelements.com
- Disilver Sulphide – Pubchem.ncbi.nlm.nih.gov
- Silver Sulfide – Chemspider.com
- Silver sulfide (Ag2S) semiconductors – Azom.com
- Silver (I) Sulfide – Chemicalbook.com
- Is Silver Sulfide An Acid or Base? – Brainly.in
- What is Silver Sulfide? – Chemical Formula & Uses – Study.com
- Silver (I) Sulfide – Chemicalbook.com
- Silver sulfide hydrochloric acid ag 2 s 2hcl 2agcl h – Coursehero.com
- The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared – Ncbi.nlm.nih.gov
- Chemical decomposition of silver sulfide? – Answers.yahoo.com
- Exposure-Related Health Effects of Silver and Silver Compounds: A Review – Academic.oup.com
- Finishing techniques in metalwork – Philamuseum.org
- Silver and sulfur: case studies, physics and possible solutions- Pdfs.semanticscholar.org

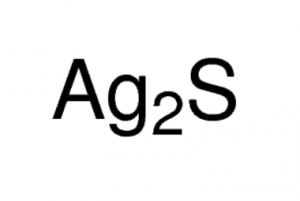
Ag2S + 2HCl = 2AgCl + H2S
How can this reaction take place despite silver being less reactive than hydrogen in the reactivity series?
Silver alone cannot displace hydrogen from hydrochloric acid, but silver sulfide can. So, this reaction is possible.