Oxime
Table of Contents
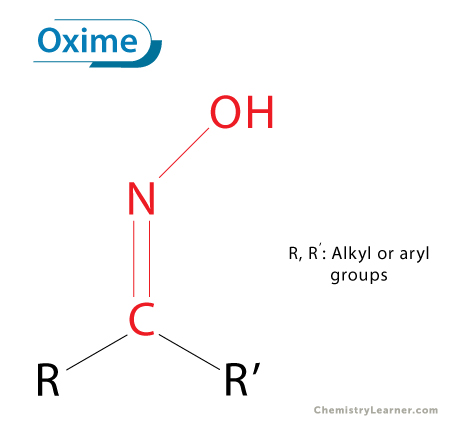
An oxime is a class of organic compounds characterized by the presence of the C=NOH functional group. The general formula is RR′C=NOH, where R and R′ can be hydrogen, alkyl, or aryl groups. Oximes are usually formed when a carbonyl compound, either an aldehyde or a ketone, reacts with hydroxylamine (NH2OH). [1-4]
Oximes are essential in both research and industry. In laboratories, they are widely used for the identification and characterization of aldehydes and ketones. In industrial chemistry, they serve as valuable intermediates. A well-known example is cyclohexanone oxime, which undergoes rearrangement to form caprolactam, the key precursor for Nylon-6.
Structure and Bonding [4]
- Functional Group: Oximes contain the C=NOH group, where carbon is double-bonded to nitrogen, and the nitrogen atom bears a hydroxyl group.
- Hybridization and Geometry: Both carbon and nitrogen atoms are largely sp2-hybridized, resulting in a nearly planar geometry.
- Bond Strength and Polarity: The C=N bond is shorter and stronger than a single C–N bond and is polar in nature.
- Hydrogen Bonding: The –OH group participates in hydrogen bonding, which strongly influences melting and boiling points.
Types of Oxime
Oximes are generally classified according to the carbonyl compound from which they are derived: [1,5]
- Alldoxime is derived from aldehyde. General formula: R–CH=NOH
- Ketoxime is derived from ketone. General formula: RR’–C=NOH
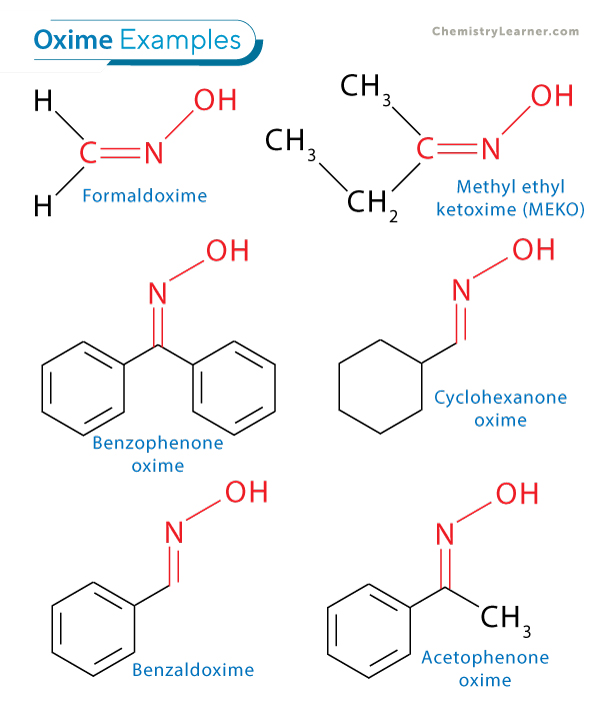
| Type | Common Name | Formula | Uses |
|---|---|---|---|
| Aldoxime | Formaldoxime | CH2=NOH | Starting material in synthesis |
| Aldoxime | Benzaldoxime | C6H5CH=NOH | Used to identify aldehydes |
| Ketoxime | Benzophenone oxime | (C6H5)2C=NOH | Intermediate for organic synthesis |
| Ketoxime | Cyclohexanone oxime | C6H10=NOH | Precursor for Nylon-6 |
| Ketoxime | Acetophenone oxime | C6H5C=NOHCH3 | Used in pharmaceuticals and chemicals |
| Ketoxime | Methyl ethyl ketoxime (MEKO) | CH3–C=NOH–C2H5 | Prevents skin formation in paints |
Physical Properties [5,6]
- State and color: Mostly white or colorless crystals. Some are thick, colorless liquids.
- Solubility: Exhibit low solubility in water but dissolve readily in polar organic solvents like ethanol and ether.
- Melting/boiling points: Melting and boiling points vary significantly depending on the specific oxime’s structure.
- IR spectra: Oximes display characteristic absorption peaks in their infrared (IR) spectra: ~3600 cm−1 for the O-H group, ~1665 cm−1 for the C=N double bond, and ~945 cm−1 for the N-O bond.
Preparation
The most common method of preparing oximes is through the condensation of aldehydes or ketones with hydroxylamine (NH2OH). In this reaction, the oxygen atom of the carbonyl group (>C=O) is replaced by the =NOH group. [2,3,5]
General Reaction
RR’C=O + NH2OH ⟶ RR’C=NOH + H2O
If R = H, then aldoxime is formed. If R and R’ are alkyl/aryl groups, then ketoxime is formed.
Examples
1. Acetone oxime is prepared by treating acetone with hydroxylamine solution in the presence of sodium acetate:
(CH3)2C=O + NH2OH ⟶ (CH3)2C=NOH + H2O
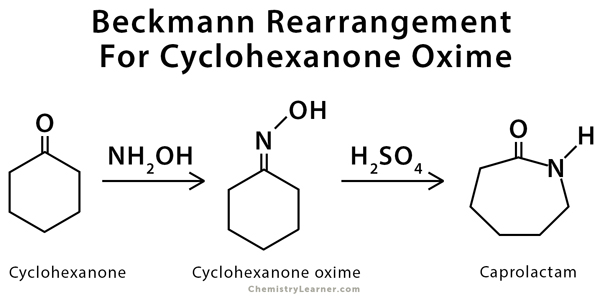
2. Cyclohexanone oxime is produced industrially by reaction of cyclohexanone with hydroxylamine:
C6H10O + NH2OH ⟶ C6H11NOH + H2O
This oxime is later used to make caprolactam for Nylon-6.
3. Benzaldoxime is obtained from benzaldehyde and hydroxylamine:
C6H5CHO + NH2OH ⟶ C6H5CH=NOH + H2O
It exists in two isomeric forms (syn and anti).
Reactions [7,8]
1. Hydrolysis
Oximes can undergo hydrolysis under acidic conditions (HCl or H2SO4) to regenerate the parent aldehyde or ketone along with hydroxylamine.
RR’C=NOH + H2O → RR’C=O + NH2OH (in presence of H2SO4)
Example: Benzaldoxime (C6H5CH=NOH) → Benzaldehyde (C6H5CHO)
2. Reduction
When reduced by an agent such as lithium aluminum hydride (LiAlH4), oximes are converted into primary amines.
RR’C=NOH → RR’CH–NH2 + NH2OH (in presence of LiAlH4)
Example: Acetoxime (CH3C=NOHCH3) → Isopropylamine (CH3CH(NH2)CH3)
3. Beckmann Rearrangement
In the presence of strong acids (e.g., H2SO4), oximes undergo the Beckmann rearrangement, producing amides or lactams.
RR’C=NOH → R–CO–NR’H (in presence of H2SO4)
Example: Cyclohexanone oxime (C6H10=NOH) → Caprolactam ((CH2)5CONH)
Overall, oximes are valuable because they link fundamental organic chemistry with practical applications, ranging from materials production to medicine. Their unique reactivity makes them indispensable in transforming simple molecules into compounds of industrial and pharmaceutical importance.