Diatomic Molecules
A molecule made up of two atoms is called a diatomic molecule. The atoms can be from the same element or different elements. A diatomic molecule has a linear geometry, meaning the atoms are connected in a straight line. Almost all diatomic molecules are gases at room temperature [1-4].
Types of Diatomic Molecules
There are two types of diatomic molecules – homonuclear and heteronuclear [1-4].
Homonuclear
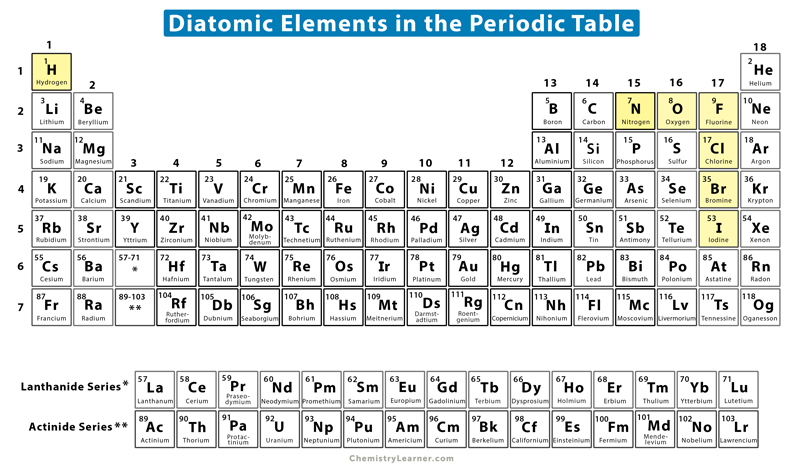
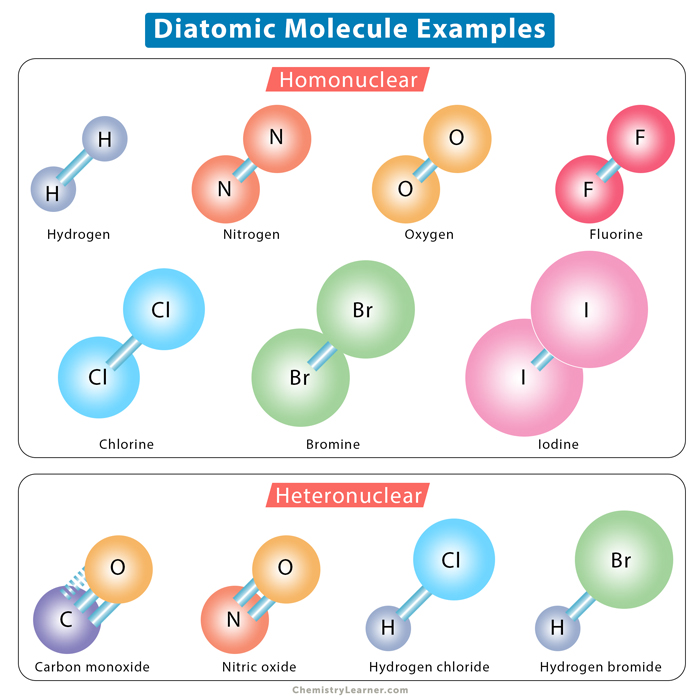
A diatomic molecule is said to be homonuclear if the two atoms are the same type. It takes form A2. The subscript 2 indicates that two atoms have combined to form the molecule through a covalent bond. The covalent bond is always nonpolar and can be single, double, or triple. A homonuclear diatomic molecule is also known as a diatomic element. There are seven of them in the periodic table.
Examples
All but bromine and iodine are gases at room temperature. Br2 is liquid, and I2 is solid at room temperature.
How to Remember the Diatomic Elements
All seven diatomic elements are nonmetals and end with ‘gen’. Hydrogen, nitrogen, and oxygen end with ‘gen’; the remaining four are halogens. An easy-to-remember mnemonic for the diatomic elements is: Have No Fear Of Ice Cold Beer.
Heteronuclear
A diatomic molecule is heteronuclear if the two atoms are of different types. The form of this molecule is A-B. Here, at least one of the atoms is a nonmetal. The bonding in a heteronuclear diatomic molecule is either covalent or coordinate covalent.
Examples
- Carbon monoxide (CO)
- Nitric oxide (NO)
- Hydrogen fluoride (HF)
- Hydrogen chloride (HCl)
- Hydrogen bromide (HBr)






it’s great mechanism and my kids use it all the time