Einsteinium
What is Einsteinium
Einsteinium (pronounced as ine-STINE-ee-em) is a radioactive metal, belonging to the family of transuranium elements, and denoted by the chemical symbol Es. It has 16 isotopes out of which einsteinium-252 is the most stable one with a half-life of 47.1 days [2, 3].
History of Einsteinium
Origin of its Name
The element is named after the renowned German physicist, Albert Einstein [1, 2].
Who Discovered Einsteinium
In 1952, a team of scientists led by American nuclear scientist Albert Ghiorso, discovered it [2].
When and Where was it Discovered
On 1st November 1952, a thermonuclear bomb explosion had been conducted on a small island near the Pacific Coast. After which, the residual radioactive material collected from the neighboring atoll was sent to Berkeley, California for examination. After a month of thorough analysis of the debris, done by Ghiorso and his teammates Stanley Thompson, Gregory Choppin, and Bernard Harvey, 200 atoms of einsteinium were discovered. Einsteinium-253 with a half-life of about 20 days was the isotope found from the debris that formed during the explosion when Uranium-238 underwent bombardment with neutrons and went through a chain of several decay reactions. However, nothing was officially revealed until 1955, due to security reasons [2, 4].
Classification and Position of Einsteinium on the Periodic Table [2]
| Group Number | Unknown |
| Group Name | Actinides |
| Period | 7 |
| Block | f |
Properties of Einsteinium [1, 2, 3]
General Properties |
||
| Relative Atomic Mass | 252 | |
Physical Properties |
||
| Color/Appearance | Unknown | |
| Luster | Unknown | |
| Odor | Unknown | |
| Melting Point/Freezing Point | 860°C (1580°F) | |
| Boiling Point | Unknown | |
| Density | Unknown | |
| State of Matter at Room Temperature (solid/liquid/gas) | Solid | |
| Hardness | Unknown | |
| Electrical Conductivity | Unknown | |
| Thermal (Heat) Conductivity | Unknown | |
Chemical Properties |
||
| Flammability | Unknown | |
| Oxidation state/Oxidation number | +2, +3 | |
Atomic Data of the Element [1, 2, 3]
| Atomic Number | 99 | ||||||
| Valence Electrons | 2 | ||||||
| Quantum Numbers | |||||||
| – n | 5 | ||||||
| – ℓ | 3 | ||||||
| – m ℓ | 0 | ||||||
| – m s | -1/2 | ||||||
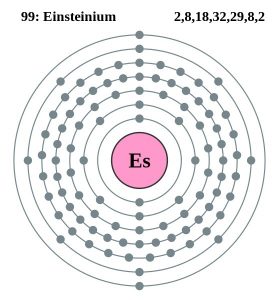
| Electron Configuration (Noble Gas Configuration) | [Rn] 5f117s2 | ||||||
| Atomic Structure | |||||||
| – Number of Electrons | 99 | ||||||
| – Number of Neutrons | 153 | ||||||
| – Number of Protons | 99 | ||||||
| Radius of Atom | |||||||
| – Atomic Radius | 2.45 Å | ||||||
| – Covalent Radius | 1.65 Å | ||||||
| Electronegativity | Unknown | ||||||
| Ionization Energy
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 619.44 | 1158 | – | – | – | – | – | |
Einsteinium Bohr Model
What is Einsteinium Used for
Apart from basic scientific research, related to study of its properties, and production of other elements with a higher atomic number, Es has no commercial applications [2, 5].
Interesting Facts
- The element was casually referred to as pandamonium by the team of discoverers before getting its official name, as the bomb testing experiment was code-named Project Panda [5].
- The symbol of einsteinium was initially proposed as E, but IUPAC changed it to Es [5].
- In 1955, the element was used to synthesize the first sample of the mendelevium [3].
Cost of Einsteinium
Since the element is synthetically produced in minute amounts, it cannot be found outside laboratory production.
- References
- https://education.jlab.org/itselemental/ele099.html
- http://www.rsc.org/periodic-table/element/99/einsteinium
- https://www.chemicool.com/elements/einsteinium.html
- https://study.com/academy/lesson/einsteinium-element-discovery-name-properties.html
- https://www.thoughtco.com/einsteinium-facts-element-99-or-es-4126476