Francium
What is Francium
Francium (pronounced as FRAN-see-em) is an alkali metal denoted by the symbol, Fr. No more than 15 grams of the element is found in the earth’s crust [1, 5].
Francium Isotopes
Its most stable isotope is Francium-223 having a half-life of 22 minutes that either undergo beta decay or alpha decay to form radium-223 and astatine-219, respectively [3].
History of Francium
How Did it Get its Name
The element has taken its name from the country, France [1].
Who Discovered Francium
It was discovered by French physicist Margaret Perey in 1939 [1].
When and How was it Discovered
Dmitri Mendeleev, the Russian chemist who first created the periodic table, had suggested the existence of an element similar to caesium yet to be discovered. After which, there were several claims, denials, and counterclaims coming from scientists who wanted to declare themselves as the first discoverer of this new element. Such claims continued to happen during the 1920’s and 1930’s based on the discovery of undetected radioactivity in some minerals and new lines in their X-ray spectra. However, when they were verified, no evidence of the suspected element was found.
It was only in 1939 when Perey, at the Curie Institute, Paris, purified the element actium to free it from radioactive impurities but still found some radioactivity in it due to the presence of the still-undiscovered francium. Many challenged her findings, and it was only after World War 2 that she was finally considered as the original discoverer of francium [1].
Classification and Position of the Element on the Periodic Table [1]
| Group | 1 |
| Period | 7 |
| Block | s |
Properties and Characteristics of Francium [1, 2, 3]
General Properties |
||
| Relative Atomic Mass (Mass Number) | 223 | |
| Molar Mass | 186.207 g/mol | |
Physical Properties |
||
| Color/Appearance | Silver Gray (Unconfirmed) | |
| Flame Color | Unknown | |
| Luster | Unknown | |
| Odor | Unknown | |
| Malleability | Yes | |
| Ductility | Yes | |
| Melting Point/Freezing Point | 21°C (70°F) | |
| Boiling Point | 650°C (1202°F) | |
| Density | 21 g/cm3 | |
| State of Matter at Room Temperature | Solid | |
| Hardness (Mohs) | Unknown | |
| Electrical Conductivity (Sm-1) | Unknown | |
| Thermal (Heat) conductivity (W m-1 K-1) | 3.61 | |
| Latent Heat of Fusion (kJ/mol) | Unknown | |
Magnetic Properties |
||
| Specific Gravity | Unknown | |
| Magnetic Ordering | 1 | |
| Permeability | Unknown | |
| Susceptibility | Unknown | |
Chemical Properties |
||
| Flammability | Unknown | |
| Oxidation state/Oxidation number | +1 | |
Atomic Data of Francium [1, 2, 3]
| Atomic Number | 87 | ||||||
| Valence Electrons | 7s | ||||||
| Quantum Numbers | |||||||
| – n | 7 | ||||||
| – ℓ | 0 | ||||||
| – m ℓ | 0 | ||||||
| – m s | +1/2 | ||||||
| Electron Configuration (Noble Gas Configuration) | [Rn] 7s1 | ||||||
| Atomic Structure | |||||||
| – Number of Electrons | 87 | ||||||
| – Number of Neutrons | 136 | ||||||
| – Number of Protons | 87 | ||||||
| Energy Levels | |||||||
| – First Energy Level | 2 | ||||||
| – Second Energy Level | 8 | ||||||
| – Third Energy Level | 18 | ||||||
| – Fourth Energy Level | 32 | ||||||
| – Fifth Energy Level | 18 | ||||||
| – Sixth Energy Level | 8 | ||||||
| – Seventh Energy Level | 1 | ||||||
| Radius of Atom | |||||||
| – Atomic Radius | 3.48 Å | ||||||
| – Covalent Radius | 2.42 Å | ||||||
| Electronegativity | 0.79 | ||||||
| Ionization Energy
(kJmol-1) |
1st | 2nd | 3rd | 4th | 5th | 6th | 7th |
| 392.96 | |||||||
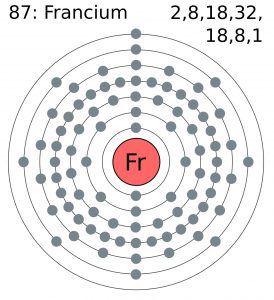
Bohr Model of Francium
Reaction of Francium in Water
Since the metal is alkaline, it is believed to react violently with water, producing francium hydroxide and hydrogen, and a massive amount of heat. So far, no such experiment has been conducted to prove it even though many false claims have been made showing francium exploding like a bomb when dropped in the ocean [4].
What is Francium Used for
There are no commercial uses of francium except in basic scientific studies due to its limited production and short half-life [1, 2].
Interesting Facts
- Among all the naturally-occurring elements, francium is the least stable one [6].
- It can be produced by bombarding thorium with protons or radium with neutrons [2].
Francium Cost
As only fewer atoms of the element have been produced in the laboratory, it is not commercially available.
- References
- http://www.rsc.org/periodic-table/element/87/francium
- https://education.jlab.org/itselemental/ele087.html
- https://www.chemicool.com/elements/francium.html
- https://www.thoughtco.com/dropping-francium-in-water-607474
- http://www.chemistryexplained.com/elements/C-K/Francium.html
- http://www.softschools.com/facts/periodic_table/francium_facts/358/









give the number of shells
There are 7 shells as can be seen from the Bohr model.
The reddish example in second picture….looks exactly like rock i have that exploded (not violently) it also retains heat and can feel a weird energy around it when hot outside
If so much is unknown then what are the pictures of? I have ores (rocks) identical to the top pic. I can tell you they do explode but not by dropping in cold water or even by spraying the rock or the water has to be warm.